Patient satisfaction with return of pharmacogenomic results utilizing a patient portal message
- PMID: 37125619
- PMCID: PMC10318570
- DOI: 10.2217/pgs-2023-0032
Patient satisfaction with return of pharmacogenomic results utilizing a patient portal message
Abstract
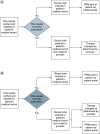
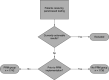
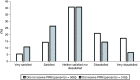
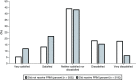
Background: Returning pharmacogenomics (PGx) results to patients is complex and challenging. Patients prefer provider education; however, a gap in provider comfort in PGx results has been documented. Objectives: This study's purpose was to evaluate satisfaction with the return of PGx test results using a patient portal message. Methods: A survey was sent to two cohorts with PGx results, one that received a PGx result message and one that did not. Results: Following implementation of the PGx result message, there was a decrease in patients reporting negative responses surrounding satisfaction in the return of their PGx results, with 39% responding negatively pre-implementation and 21% post-implementation. Conclusion: Satisfaction with the return of results improved following the implementation of a patient portal message.
Keywords: humans; patient satisfaction; personal satisfaction; pharmacists; pharmacogenetics; surveys and questionnaires.
Conflict of interest statement
The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Figures
References
-
- National Institute of General Medical Sciences. Pharmacogenomics. https://nigms.nih.gov/education/fact-sheets/Pages/pharmacogenomics.aspx
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
