Orthogonal Light-Activated DNA for Patterned Biocomputing within Synthetic Cells
- PMID: 37125650
- PMCID: PMC10161232
- DOI: 10.1021/jacs.3c02350
Orthogonal Light-Activated DNA for Patterned Biocomputing within Synthetic Cells
Abstract
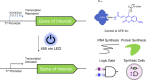
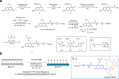
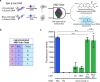
Cell-free gene expression is a vital research tool to study biological systems in defined minimal environments and has promising applications in biotechnology. Developing methods to control DNA templates for cell-free expression will be important for precise regulation of complex biological pathways and use with synthetic cells, particularly using remote, nondamaging stimuli such as visible light. Here, we have synthesized blue light-activatable DNA parts that tightly regulate cell-free RNA and protein synthesis. We found that this blue light-activated DNA could initiate expression orthogonally to our previously generated ultraviolet (UV) light-activated DNA, which we used to generate a dual-wavelength light-controlled cell-free AND-gate. By encapsulating these orthogonal light-activated DNAs into synthetic cells, we used two overlapping patterns of blue and UV light to provide precise spatiotemporal control over the logic gate. Our blue and UV orthogonal light-activated DNAs will open the door for precise control of cell-free systems in biology and medicine.
Conflict of interest statement
The authors declare no competing financial interest.
Figures


References
-
- Garenne D.; Haines M. C.; Romantseva E. F.; Freemont P.; Strychalski E. A.; Noireaux V. Cell-Free Gene Expression. Nat. Rev. Methods Primers 2021, 1, 4910.1038/s43586-021-00046-x. - DOI
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

