Enantioselective Synthesis of Fluorinated Indolizidinone Derivatives
- PMID: 37125898
- PMCID: PMC10186376
- DOI: 10.1021/acs.orglett.3c00903
Enantioselective Synthesis of Fluorinated Indolizidinone Derivatives
Abstract
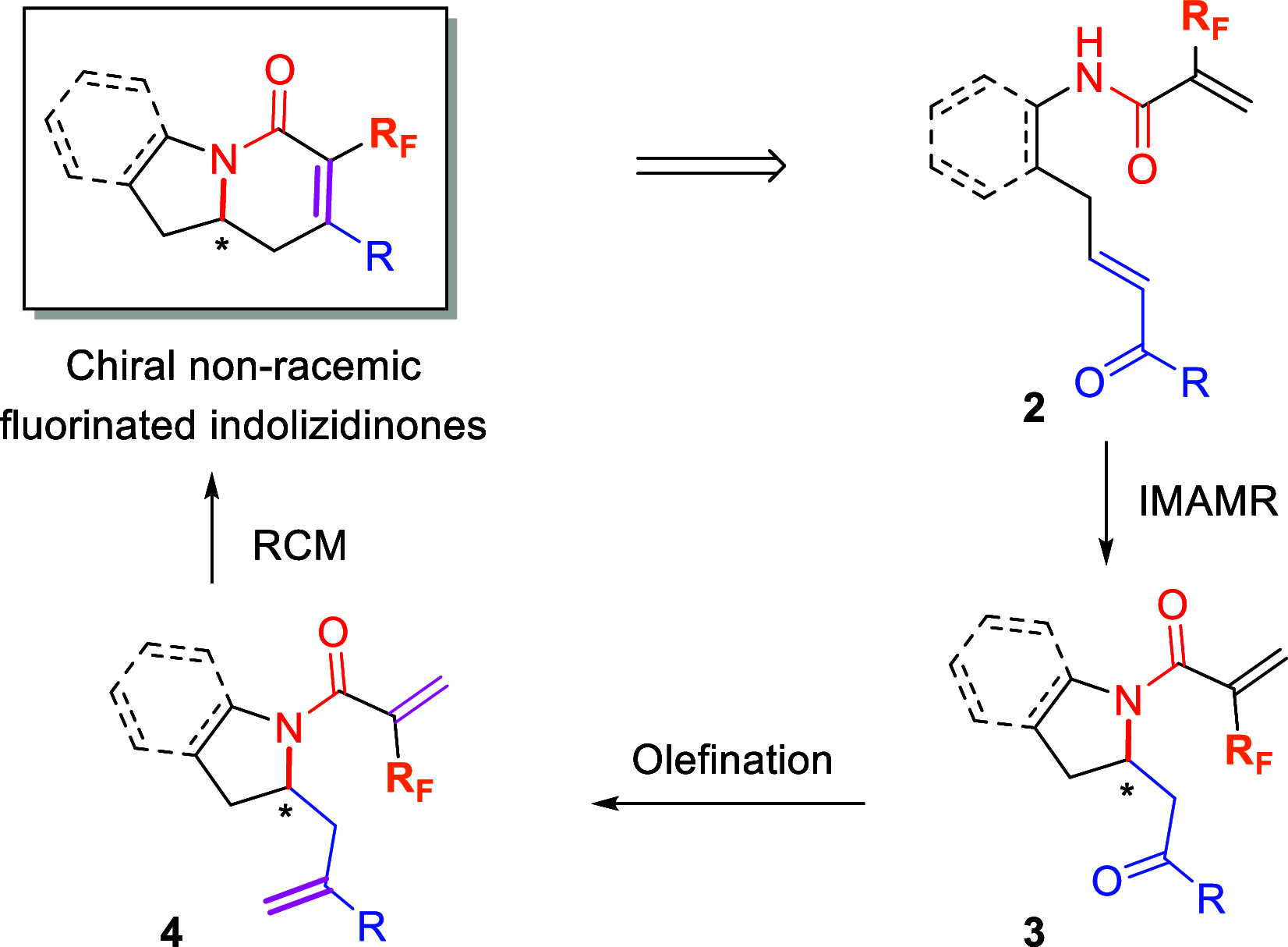
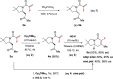
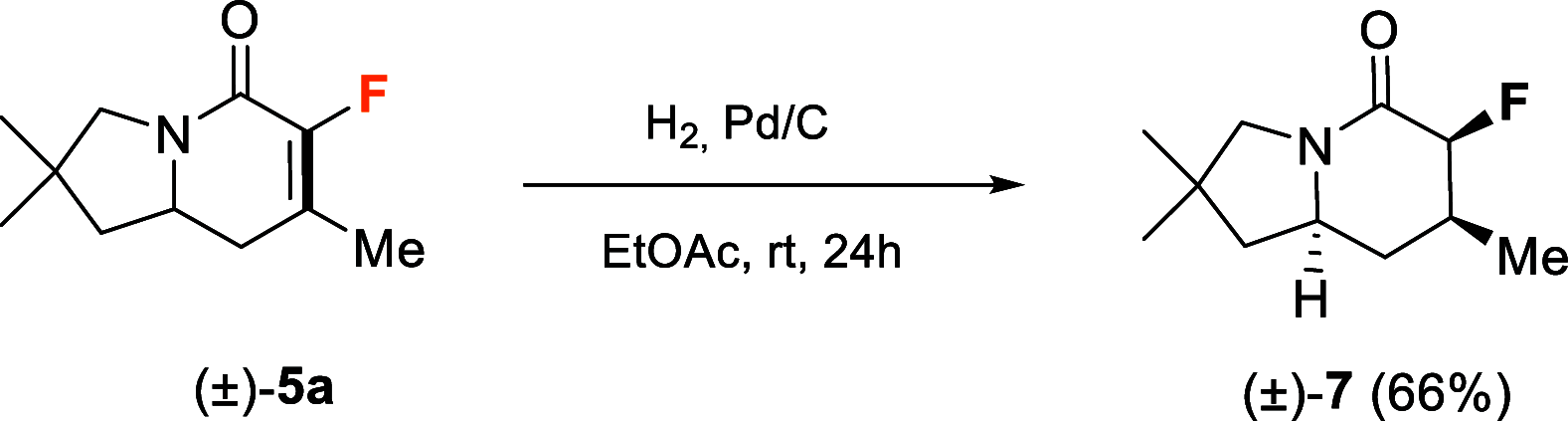
The enantioselective synthesis of fluorinated indolizidinone derivatives has been developed. The process involved an enantioselective intramolecular aza-Michael reaction of conjugated amides bearing a pendant α,β-unsaturated ketone moiety, catalyzed by the (S)-TRIP-derived phosphoric acid, followed by dimethyltitanocene methylenation and ring closing metathesis (RCM). Final indolizidine-derived products comprise a fluorine-containing tetrasubstituted double bond generated by the RCM reaction, which is a challenging task. The whole synthetic sequence took place in acceptable overall yields with excellent enantioselectivities.
Conflict of interest statement
The authors declare no competing financial interest.
Figures



References
-
- Zhang J.; Morris-Natschke S. L.; Ma D.; Shang X. F.; Yang C. J.; Liu Y. Q.; Lee K. H. Biologically Active Indolizidine Alkaloids. Med. Res. Rev. 2021, 41, 928.10.1002/med.21747. - DOI - PubMed
- Sharma V.; Kamal R.; Kumar D.; Kumar V. Indolizidine Alkaloids. Prospective Lead Molecules in Medicinal Chemistry. Curr. Tradit. Med. 2021, 7, 45.10.2174/2215083805666190617145228. - DOI
- Kim E.; Lee Y.; Lee S.; Park S. B. Discovery, Understanding, and Bioapplication of Organic Fluorophore: a Case Study with an Indolizine-Based Novel Fluorophore, Seoul-fluor. Acc. Chem. Res. 2015, 48, 538.10.1021/ar500370v. - DOI - PubMed
- Sharma V.; Kumar V. Indolizine: a Biologically Active Moiety. Med. Chem. Res. 2014, 23, 3593.10.1007/s00044-014-0940-1. - DOI
- Singh G. S.; Mmatli E. E. Recent Progress in Synthesis and Bioactivity Studies of Indolizines. Eur. J. Med. Chem. 2011, 46, 5237.10.1016/j.ejmech.2011.08.042. - DOI - PubMed
-
-
For recent reviews of the synthesis of indolizidine scaffolds, see:
- Frǎnová P.; Marchalín Š. Recent Developments in the Synthesis of Polyhydroxylated Indolizidines. Eur. J. Org. Chem. 2022, 2022, e20220074210.1002/ejoc.202200742. - DOI
- Ratmanova N. K.; Andreev I. A.; Leontiev A. V.; Momotova D.; Novoselov A. M.; Ivanova O. A.; Trushkov I. V. Strategic Approaches to the Synthesis of Pyrrolizidine and Indolizidine Alkaloids. Tetrahedron 2020, 76, 131031.10.1016/j.tet.2020.131031. - DOI
- Nájera C.; Sansano J. M. Synthesis of Pyrrolizidines and Indolizidines by Multicomponent 1,3-Dipolar Cycloaddition of Azomethine Ylides. Pure Appl. Chem. 2019, 91, 575.10.1515/pac-2018-0710. - DOI
- Davies S. G.; Fletcher A. M.; Roberts P. M.; Thomson J. E. Asymmetric Synthesis of Pyrrolizidines, Indolizidines and Quinolizidines via a Double Reductive Cyclisation Protocol. Synlett 2017, 28, 2697.10.1055/s-0036-1590975. - DOI
- Sadowski B.; Klajn J.; Gryko D. T. Recent Advances in the Synthesis of Indolizines and their π-Expanded Analogues. Org. Biomol. Chem. 2016, 14, 7804.10.1039/C6OB00985A. - DOI - PubMed
- Michael J. P.Chapter One - Simple Indolizidine and Quinolizidine Alkaloids. In The Alkaloids: Chemistry and Biology; Knölker H.-J., Ed.; Academic Press, 2016; Vol. 75, pp 1–498. - PubMed
- Michael J. P. Indolizidine and Quinolizidine Alkaloids. Nat. Prod. Rep. 2008, 25, 139.and references cited therein10.1039/B612166G. - DOI - PubMed
-
-
- Meanwell N. A. Fluorine and Fluorinated Motifs in the Design and Application of Bioisosteres for Drug Design. J. Med. Chem. 2018, 61, 5822.10.1021/acs.jmedchem.7b01788. - DOI - PubMed
- Gillis E. P.; Eastman K. J.; Hill M. D.; Donnelly D. J.; Meanwell N. A. Applications of Fluorine in Medicinal Chemistry. J. Med. Chem. 2015, 58, 8315.10.1021/acs.jmedchem.5b00258. - DOI - PubMed
- Swallow S. Fluorine in medicinal chemistry. Prog. Med. Chem. 2015, 54, 65.10.1016/bs.pmch.2014.11.001. - DOI - PubMed
- Purser S.; Moore P. R.; Swallow S.; Gouverneur V. Fluorine in Medicinal Chemistry. Chem. Soc. Rev. 2008, 37, 320.10.1039/B610213C. - DOI - PubMed
-
- Zhang C. Fluorine in Medicinal Chemistry. In Perspective to COVID-19. ACS Omega 2022, 7, 18206.10.1021/acsomega.2c01121. - DOI - PMC - PubMed
- Mei H.; Han J.; Fustero S.; Medio-Simon M.; Sedgwick D. M.; Santi C.; Ruzziconi R.; Soloshonok V. A. Frontispiece: Fluorine-Containing Drugs Approved by the FDA in 2018. Chem. - Eur. J. 2019, 25, 840.10.1002/chem.201985161. - DOI - PubMed
- Zhou Y.; Wang J.; Gu Z.; Wang S.; Zhu W.; Acena J. L.; Soloshonok V. A.; Izawa K.; Liu H. Next Generation of Fluorine-Containing Pharmaceuticals, Compounds Currently in Phase II-III Clinical Trials of Major Pharmaceutical Companies: New Structural Trends and Therapeutic Areas. Chem. Rev. 2016, 116, 422.10.1021/acs.chemrev.5b00392. - DOI - PubMed
- Wang J.; Sanchez-Rosello M.; Acena J. L.; del Pozo C.; Sorochinsky A. E.; Fustero S.; Soloshonok V. A.; Liu H. Fluorine in Pharmaceutical Industry: Fluorine-Containing Drugs Introduced to the Market in the Last Decade (2001–2011). Chem. Rev. 2014, 114, 2432.10.1021/cr4002879. - DOI - PubMed
-
- O’Hagan D. Fluorine in Health Care: Organofluorine Containing Blockbuster Drugs. J. Fluorine Chem. 2010, 131, 1071.10.1016/j.jfluchem.2010.03.003. - DOI
LinkOut - more resources
Full Text Sources

