Evaluating cancer genetic services in a safety net system: overcoming barriers for a lasting impact beyond the CHARM research project
- PMID: 37126135
- PMCID: PMC10271961
- DOI: 10.1007/s12687-023-00647-x
Evaluating cancer genetic services in a safety net system: overcoming barriers for a lasting impact beyond the CHARM research project
Abstract
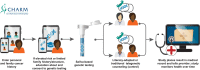
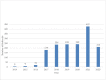
Underserved patients face substantial barriers to receiving cancer genetic services. The Cancer Health Assessments Reaching Many (CHARM) study evaluated ways to increase access to genetic testing for individuals in underserved populations at risk for hereditary cancer syndromes (HCS). Here, we report the successful implementation of CHARM in a low-resource environment and the development of sustainable processes to continue genetic risk assessment in this setting. The research team involved key clinical personnel and patient advisors at Denver Health to provide input on study methods and materials. Through iterative and collaborative stakeholder engagement, the team identified barriers and developed solutions that would both facilitate participation in CHARM and be feasible to implement and sustain long term in clinical care. With a focus on infrastructure building, educational modules were developed to increase awareness among referring providers, and standard methods of identifying and managing HCS patients were implemented in the electronic medical record. Three hundred sixty-four DH patients successfully completed the risk assessment tool within the study, and we observed a sustained increase in referrals to genetics for HCS (from 179 in 2017 to 427 in 2021 post-intervention). Implementation of the CHARM study at a low-resourced safety net health system resulted in sustainable improvements in access to cancer genetic risk assessment and services that continue even after the study ended.Trial registration NCT03426878.
Keywords: Barriers; Genetic services; Research collaboration; Research implementation.
© 2023. The Author(s).
Conflict of interest statement
Sonia Okuyama, Larissa L White, Katherine P Anderson, Elizabeth Medina, Michael C. Leo, Joanna E. Buckley, Katrina Goddard, Chelese Ransom, Paige Jackson, Tia L. Kauffman, Benjamin S Wilfond, and Heather S. Feigelson declare that they have no conflict of interest.
Kathleen F. Mittendorf discloses salary support from NIH/NCI for the development of the PREMM model (R01CA132829, PI: Syngal; role: co-investigator), salary support from NIH/NCI (U01CA232829; MPIs: Wiesner & Orlando; role: study personnel), and salary support from NIH/NHGRI (U01HG011181; MPIs Roden, Velez Edwards, & Wei; role: study personnel) for studies involving the implementation of the MeTree family history tool including in safety-net settings and salary support from funds awarded to their institution by GE Healthcare (PI: Park; role: study personnel).
Figures
References
-
- Amendola LM, Berg JS, Horowitz CR, Angelo F, Bensen JT, Biesecker BB, Biesecker LG, Cooper GM, East K, Filipski K, Fullerton SM, Gelb BD, Goddard KAB, Hailu B, Hart R, Hassmiller-Lich K, Joseph G, Kenny EE, Koenig BA, Knight S, Kwok PY, Lewis KL, McGuire AL, Norton ME, Ou J, Parsons DW, Powell BC, Risch N, Robinson M, Rini C, Scollon S, Slavotinek AM, Veenstra DL, Wasserstein MP, Wilfond BS, Hindorff LA, Plon SE, Jarvik GP. The Clinical Sequencing Evidence-Generating Research Consortium: integrating genomic sequencing in diverse and medically underserved populations. Am J Hum Genet. 2018;103:319–327. doi: 10.1016/j.ajhg.2018.08.007. - DOI - PMC - PubMed
-
- Barger S, Sullivan SD, Bell-Brown A, Bott B, Ciccarella AM, Golenski J, Gorman M, Johnson J, Kreizenbeck K, Kurttila F, Mason G, Myers J, Seigel C, Wade JL, 3rd, Walia G, Watabayashi K, Lyman GH, Ramsey SD. Effective stakeholder engagement: design and implementation of a clinical trial (SWOG S1415CD) to improve cancer care. BMC Med Res Methodol. 2019;19:119. doi: 10.1186/s12874-019-0764-2. - DOI - PMC - PubMed
Associated data
LinkOut - more resources
Full Text Sources
Medical