Contribution of Somatic Ras/Raf/Mitogen-Activated Protein Kinase Variants in the Hippocampus in Drug-Resistant Mesial Temporal Lobe Epilepsy
- PMID: 37126322
- PMCID: PMC10152377
- DOI: 10.1001/jamaneurol.2023.0473
Contribution of Somatic Ras/Raf/Mitogen-Activated Protein Kinase Variants in the Hippocampus in Drug-Resistant Mesial Temporal Lobe Epilepsy
Abstract
Importance: Mesial temporal lobe epilepsy (MTLE) is the most common focal epilepsy subtype and is often refractory to antiseizure medications. While most patients with MTLE do not have pathogenic germline genetic variants, the contribution of postzygotic (ie, somatic) variants in the brain is unknown.
Objective: To test the association between pathogenic somatic variants in the hippocampus and MTLE.
Design, setting, and participants: This case-control genetic association study analyzed the DNA derived from hippocampal tissue of neurosurgically treated patients with MTLE and age-matched and sex-matched neurotypical controls. Participants treated at level 4 epilepsy centers were enrolled from 1988 through 2019, and clinical data were collected retrospectively. Whole-exome and gene-panel sequencing (each genomic region sequenced more than 500 times on average) were used to identify candidate pathogenic somatic variants. A subset of novel variants was functionally evaluated using cellular and molecular assays. Patients with nonlesional and lesional (mesial temporal sclerosis, focal cortical dysplasia, and low-grade epilepsy-associated tumors) drug-resistant MTLE who underwent anterior medial temporal lobectomy were eligible. All patients with available frozen tissue and appropriate consents were included. Control brain tissue was obtained from neurotypical donors at brain banks. Data were analyzed from June 2020 to August 2022.
Exposures: Drug-resistant MTLE.
Main outcomes and measures: Presence and abundance of pathogenic somatic variants in the hippocampus vs the unaffected temporal neocortex.
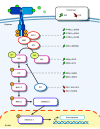
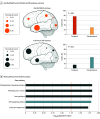
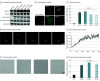
Results: Of 105 included patients with MTLE, 53 (50.5%) were female, and the median (IQR) age was 32 (26-44) years; of 30 neurotypical controls, 11 (36.7%) were female, and the median (IQR) age was 37 (18-53) years. Eleven pathogenic somatic variants enriched in the hippocampus relative to the unaffected temporal neocortex (median [IQR] variant allele frequency, 1.92 [1.5-2.7] vs 0.3 [0-0.9]; P = .01) were detected in patients with MTLE but not in controls. Ten of these variants were in PTPN11, SOS1, KRAS, BRAF, and NF1, all predicted to constitutively activate Ras/Raf/mitogen-activated protein kinase (MAPK) signaling. Immunohistochemical studies of variant-positive hippocampal tissue demonstrated increased Erk1/2 phosphorylation, indicative of Ras/Raf/MAPK activation, predominantly in glial cells. Molecular assays showed abnormal liquid-liquid phase separation for the PTPN11 variants as a possible dominant gain-of-function mechanism.
Conclusions and relevance: Hippocampal somatic variants, particularly those activating Ras/Raf/MAPK signaling, may contribute to the pathogenesis of sporadic, drug-resistant MTLE. These findings may provide a novel genetic mechanism and highlight new therapeutic targets for this common indication for epilepsy surgery.
Conflict of interest statement
Figures

Comment in
-
Oncogenic Pathways Provide Clue to the Etiology of Human Mesial Temporal Lobe Epilepsy.JAMA Neurol. 2023 Jun 1;80(6):546-547. doi: 10.1001/jamaneurol.2023.0465. JAMA Neurol. 2023. PMID: 37126324 No abstract available.
-
Open Sesame: Door to Enriched Somatic Variants Underlying Sporadic mTLE.Epilepsy Curr. 2023 Sep 28;23(6):383-385. doi: 10.1177/15357597231201127. eCollection 2023 Nov-Dec. Epilepsy Curr. 2023. PMID: 38269352 Free PMC article.
Comment on
-
Oncogenic Pathways Provide Clue to the Etiology of Human Mesial Temporal Lobe Epilepsy.JAMA Neurol. 2023 Jun 1;80(6):546-547. doi: 10.1001/jamaneurol.2023.0465. JAMA Neurol. 2023. PMID: 37126324 No abstract available.
References
-
- Lamberink HJ, Otte WM, Blümcke I, Braun KPJ; European Epilepsy Brain Bank Writing Group; Study Group; European Reference Network EpiCARE . Seizure outcome and use of antiepileptic drugs after epilepsy surgery according to histopathological diagnosis: a retrospective multicentre cohort study. Lancet Neurol. 2020;19(9):748-757. doi: 10.1016/S1474-4422(20)30220-9 - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- L30 NS118655/NS/NINDS NIH HHS/United States
- DP2 AG072437/AG/NIA NIH HHS/United States
- HHMI/Howard Hughes Medical Institute/United States
- R01 NS111029/NS/NINDS NIH HHS/United States
- T32 GM007753/GM/NIGMS NIH HHS/United States
- K08 NS128272/NS/NINDS NIH HHS/United States
- R01 NS117609/NS/NINDS NIH HHS/United States
- R01 NS035129/NS/NINDS NIH HHS/United States
- R25 NS065743/NS/NINDS NIH HHS/United States
- R01 AG070921/AG/NIA NIH HHS/United States
- 2022032/DDCF/Doris Duke Charitable Foundation/United States
- P50 HD105351/HD/NICHD NIH HHS/United States
- UL1 TR001863/TR/NCATS NIH HHS/United States
- R01 NS094596/NS/NINDS NIH HHS/United States
- R01 NS109358/NS/NINDS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

