Effect of an Herbal-Based Injection on 28-Day Mortality in Patients With Sepsis: The EXIT-SEP Randomized Clinical Trial
- PMID: 37126332
- PMCID: PMC10152378
- DOI: 10.1001/jamainternmed.2023.0780
Effect of an Herbal-Based Injection on 28-Day Mortality in Patients With Sepsis: The EXIT-SEP Randomized Clinical Trial
Abstract
Importance: Previous research has suggested that Xuebijing injection (XBJ), an herbal-based intravenous preparation, may reduce mortality among patients with sepsis.
Objective: To determine the effect of XBJ vs placebo on 28-day mortality among patients with sepsis.
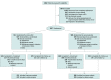
Design, setting, and participants: The Efficacy of Xuebijing Injection in Patients With Sepsis (EXIT-SEP) trial was a multicenter, randomized double-blind, placebo-controlled trial conducted in intensive care units at 45 sites and included 1817 randomized patients with sepsis (sepsis 3.0) present for less than 48 hours. Patients aged 18 to 75 years with a Sequential Organ Failure Assessment score of 2 to 13 were enrolled. The study was conducted from October 2017 to June 2019. The final date of follow-up was July 26, 2019. Data analysis was performed from January 2020 to August 2022.
Interventions: The patients were randomized to receive either intravenous infusion of XBJ (100 mL, n = 911) or volume-matched saline placebo (n = 906) every 12 hours for 5 days.
Main outcomes and measures: The primary outcome was 28-day mortality.
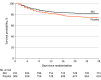
Results: Among the 1817 patients who were randomized (mean [SD] age, 56.5 [13.5] years; 1199 [66.0%] men), 1760 (96.9%) completed the trial. In these patients, the 28-day mortality rate was significantly different between the placebo group and the XBJ group (230 of 882 patients [26.1%] vs 165 of 878 patients [18.8%], respectively; P < .001). The absolute risk difference was 7.3 (95% CI, 3.4-11.2) percentage points. The incidence of adverse events was 222 of 878 patients (25.3%) in the placebo group and 200 of 872 patients (22.9%) in the XBJ group.
Conclusions and relevance: In this randomized clinical trial among patients with sepsis, the administration of XBJ reduced 28-day mortality compared with placebo.
Trial registration: ClinicalTrials.gov Identifier: NCT03238742.
Conflict of interest statement
Figures
Comment in
-
Xuebijing Injection for the Treatment of Sepsis: What Would a Path to FDA Approval Look Like?JAMA Intern Med. 2023 Jul 1;183(7):655-657. doi: 10.1001/jamainternmed.2023.0788. JAMA Intern Med. 2023. PMID: 37126325 No abstract available.
-
Efficacy of Xuebijing Injection for Sepsis (EXIT-SEP): Lost in Translation.Anaesth Crit Care Pain Med. 2023 Aug;42(4):101257. doi: 10.1016/j.accpm.2023.101257. Epub 2023 Jun 1. Anaesth Crit Care Pain Med. 2023. PMID: 37268272 No abstract available.
-
Xuebijing Injection for Sepsis Treatment: When Will It Be Approved Outside of China?JAMA Intern Med. 2023 Nov 1;183(11):1280-1281. doi: 10.1001/jamainternmed.2023.4398. JAMA Intern Med. 2023. PMID: 37721746 No abstract available.

