Fluorescence-based protocol for revealing cellular arrangement in biofilms
- PMID: 37126442
- PMCID: PMC10165451
- DOI: 10.1016/j.xpro.2023.102270
Fluorescence-based protocol for revealing cellular arrangement in biofilms
Abstract
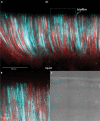
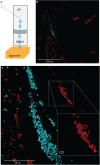
Standardized assays have greatly advanced the understanding of multicellular bacterial biofilms, but they lack cell-scale detail. Here, we present a fluorescence-based protocol that builds on past assays to reveal the cellular-scale arrangement within biofilms. We describe steps for growing biofilms on cover glass, followed by imaging and visualization of cellular arrangements in biofilms. We have applied this protocol to study Escherichia coli biofilms, though it could also be adapted to study biofilm formation in other species. For complete details on the use and execution of this protocol, please refer to Puri et al. (2023).1.
Keywords: Cell-based Assays; Microbiology; Microscopy.
Copyright © 2023. Published by Elsevier Inc.
Conflict of interest statement
Declaration of interests The authors declare no competing interests.
Figures


References
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

