Senecavirus A-induced glycolysis facilitates virus replication by promoting lactate production that attenuates the interaction between MAVS and RIG-I
- PMID: 37126525
- PMCID: PMC10174517
- DOI: 10.1371/journal.ppat.1011371
Senecavirus A-induced glycolysis facilitates virus replication by promoting lactate production that attenuates the interaction between MAVS and RIG-I
Abstract
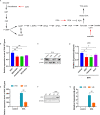
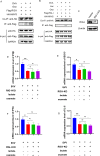
Senecavirus A (SVA)-induced porcine idiopathic vesicular disease has caused huge economic losses worldwide. Glucose metabolism in the host cell is essential for SVA proliferation; however, the impact of the virus on glucose metabolism in host cells and the subsequent effects are still unknown. Here, glycolysis induced by SVA is shown to facilitate virus replication by promoting lactate production, which then attenuates the interaction between the mitochondrial antiviral-signaling protein (MAVS) and retinoic acid-inducible gene I (RIG-I). SVA induces glycolysis in PK-15 cells, as indicated by significantly increased expression of hexokinase 2 (HK2), 6-phosphofructokinase (PFKM), pyruvate kinase M (PKM), phosphoglycerate kinase 1 (PGK1), hypoxia-inducible factor-1 alpha (HIF-1α), and superoxide dismutase-2 (SOD2) in a dose-and replication-dependent manner, and enhanced lactate production, while reducing ATP generation. Overexpression of PKM, PGK1, HIF-1α, and PDK3 in PK-15 cells and high glucose concentrations promote SVA replication, while glycolytic inhibitors decrease it. Inhibition of RLR signaling allowed better replication of SVA by promoting lactate production to attenuate the interaction between MAVS and RIG-I, and regulatory effect of glycolysis on replication of SVA was mainly via RIG-I signaling. SVA infection in mice increased expression of PKM and PGK1 in tissues and serum yields of lactate. Mice treated with high glucose and administered sodium lactate showed elevated lactate levels and better SVA replication, as well as suppressed induction of RIG-I, interferon beta (IFNβ), IFNα, interferon-stimulated gene 15 (ISG15), and interleukin 6 (IL-6). The inhibitory effect on interferons was lower in mice administered sodium oxamate and low glucose compared to the high glucose, indicating that RLR signaling was inhibited by SVA infection through lactate in vitro and in vivo. These results provide a new perspective on the relationship between metabolism and innate immunity of the host in SVA infection, suggesting that glycolysis or lactate may be new targets against the virus.
Copyright: © 2023 Li et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures







References
Publication types
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

