Bacterial catabolism of membrane phospholipids links marine biogeochemical cycles
- PMID: 37126561
- PMCID: PMC10132767
- DOI: 10.1126/sciadv.adf5122
Bacterial catabolism of membrane phospholipids links marine biogeochemical cycles
Abstract
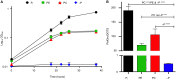
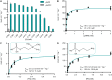
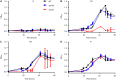
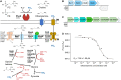
In marine systems, the availability of inorganic phosphate can limit primary production leading to bacterial and phytoplankton utilization of the plethora of organic forms available. Among these are phospholipids that form the lipid bilayer of all cells as well as released extracellular vesicles. However, information on phospholipid degradation is almost nonexistent despite their relevance for biogeochemical cycling. Here, we identify complete catabolic pathways for the degradation of the common phospholipid headgroups phosphocholine (PC) and phosphorylethanolamine (PE) in marine bacteria. Using Phaeobacter sp. MED193 as a model, we provide genetic and biochemical evidence that extracellular hydrolysis of phospholipids liberates the nitrogen-containing substrates ethanolamine and choline. Transporters for ethanolamine (EtoX) and choline (BetT) are ubiquitous and highly expressed in the global ocean throughout the water column, highlighting the importance of phospholipid and especially PE catabolism in situ. Thus, catabolic activation of the ethanolamine and choline degradation pathways, subsequent to phospholipid metabolism, specifically links, and hence unites, the phosphorus, nitrogen, and carbon cycles.
Figures

References
-
- D. M. Karl, K. M. Björkman, J. E. Dore, L. Fujieki, D. V. Hebel, T. Houlihan, R. M. Letelier, L. M. Tupas, Ecological nitrogen-to-phosphorus stoichiometry at station ALOHA. Deep Sea Res. Part II. 48, 1529–1566 (2001).
-
- W. K. W. Li, Annual average abundance of heterotrophic bacteria and Synechococcus in surface ocean waters. Limnol. Oceanogr. 43, 1746–1753 (1998).
-
- A. Buchan, G. R. LeCleir, C. A. Gulvik, J. M. González, Master recyclers: Features and functions of bacteria associated with phytoplankton blooms. Nat. Rev. Microbiol. 12, 686–698 (2014). - PubMed
-
- F. Azam, T. Fenchel, J. G. Field, J. S. Gray, L. A. Meyer-Reil, F. Thingstad, The ecological role of water-column microbes in the Sea. Mar. Ecol. Prog. Ser. 10, 257–263 (1983).
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

