Proteomics and phosphoproteomics of failing human left ventricle identifies dilated cardiomyopathy-associated phosphorylation of CTNNA3
- PMID: 37126683
- PMCID: PMC10175742
- DOI: 10.1073/pnas.2212118120
Proteomics and phosphoproteomics of failing human left ventricle identifies dilated cardiomyopathy-associated phosphorylation of CTNNA3
Abstract
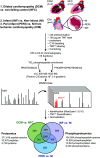
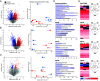
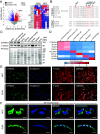
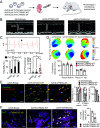
The prognosis and treatment outcomes of heart failure (HF) patients rely heavily on disease etiology, yet the majority of underlying signaling mechanisms are complex and not fully elucidated. Phosphorylation is a major point of protein regulation with rapid and profound effects on the function and activity of protein networks. Currently, there is a lack of comprehensive proteomic and phosphoproteomic studies examining cardiac tissue from HF patients with either dilated dilated cardiomyopathy (DCM) or ischemic cardiomyopathy (ICM). Here, we used a combined proteomic and phosphoproteomic approach to identify and quantify more than 5,000 total proteins with greater than 13,000 corresponding phosphorylation sites across explanted left ventricle (LV) tissue samples, including HF patients with DCM vs. nonfailing controls (NFC), and left ventricular infarct vs. noninfarct, and periinfarct vs. noninfarct regions of HF patients with ICM. Each pair-wise comparison revealed unique global proteomic and phosphoproteomic profiles with both shared and etiology-specific perturbations. With this approach, we identified a DCM-associated hyperphosphorylation cluster in the cardiomyocyte intercalated disc (ICD) protein, αT-catenin (CTNNA3). We demonstrate using both ex vivo isolated cardiomyocytes and in vivo using an AAV9-mediated overexpression mouse model, that CTNNA3 phosphorylation at these residues plays a key role in maintaining protein localization at the cardiomyocyte ICD to regulate conductance and cell-cell adhesion. Collectively, this integrative proteomic/phosphoproteomic approach identifies region- and etiology-associated signaling pathways in human HF and describes a role for CTNNA3 phosphorylation in the pathophysiology of DCM.
Keywords: bioinformatics; heart failure; intercalated disc; phosphoproteomics; signaling.
Conflict of interest statement
The authors declare no competing interest.
Figures



References
-
- G. B. o. D. S. Collaborators, Global, regional, and national incidence, prevalence, and years lived with disability for 301 acute and chronic diseases and injuries in 188 countries, 1990 to 2013: A systematic analysis for the global burden of disease study 2013. Lancet 386, 743–800 (2015). - PMC - PubMed
-
- Cook C., Cole G., Asaria P., Jabbour R., Francis D. P., The annual global economic burden of heart failure. Int. J. Cardiol. 171, 368–376 (2014). - PubMed
-
- Ambrosy A. P., et al. , The global health and economic burden of hospitalizations for heart failure: Lessons learned from hospitalized heart failure registries. J. Am. Coll. Cardiol. 63, 1123–1133 (2014). - PubMed
-
- Gajanana D., et al. , Mortality in systolic heart failure revisited: Ischemic versus non-ischemic cardiomyopathy. Int. J. Cardiol. 224, 15–17 (2016). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

