Nonpathological inflammation drives the development of an avian flight adaptation
- PMID: 37126698
- PMCID: PMC10175837
- DOI: 10.1073/pnas.2219757120
Nonpathological inflammation drives the development of an avian flight adaptation
Erratum in
-
Correction for Rashid et al., Nonpathological inflammation drives the development of an avian flight adaptation.Proc Natl Acad Sci U S A. 2025 Mar 25;122(12):e2503260122. doi: 10.1073/pnas.2503260122. Epub 2025 Mar 6. Proc Natl Acad Sci U S A. 2025. PMID: 40048267 Free PMC article. No abstract available.
Abstract
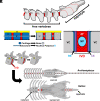
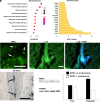
The development of modern birds provides a window into the biology of their dinosaur ancestors. We investigated avian postnatal development and found that sterile inflammation drives formation of the pygostyle, a compound structure resulting from bone fusion in the tail. Inflammation is generally induced by compromised tissue integrity, but here is involved in normal bone development. Transcriptome profiling and immuno/histochemistry reveal a robust inflammatory response that resembles bone fracture healing. The data suggest the involvement of necroptosis and multiple immune cell types, notably heterophils (the avian equivalent of neutrophils). Additionally, nucleus pulposus structures, heretofore unknown in birds, are involved in disc remodeling. Anti-inflammatory corticosteroid treatment inhibited vertebral fusion, substantiating the crucial role of inflammation in the ankylosis process. This study shows that inflammation can drive developmental skeletogenesis, in this case leading to the formation of a flight-adapted tail structure on the evolutionary path to modern avians.
Keywords: corticosteroid; heterophil; intervertebral disc; necroptosis; nucleus pulposus.
Conflict of interest statement
The authors declare no competing interest.
Figures



References
-
- Chiappe L. M., Glorified Dinosaurs: The Origin and Early Evolution of Birds (University of New South Wales or John Wiley & Sons Inc., Sydney, Australia or Hoboken, NJ USA), 2007).
-
- Wang W., O’Connor J. K., Morphological coevolution of the pygostyle and tail feathers in Early Cretaceous birds. Vertebrate PalAsiatica 55, 289–314 (2017).
-
- Barsbold R., et al. , A pygostyle from a non-avian theropod. Nature 403, 155–156 (2000). - PubMed
-
- Zhou S., Zhou Z., O’Connor J., A new piscivorous ornithuromorph from the Jehol Biota. Hist. Biol. 26, 608–618 (2014).
-
- Baumel J., Functional Morphology of the Tail Apparatus of the Pigeon (Columba livia) (Springer-Verlag, Berlin Heidelberg, ed. 1, 1988). p. 115. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

