Application of a quantitative framework to improve the accuracy of a bacterial infection model
- PMID: 37126703
- PMCID: PMC10175807
- DOI: 10.1073/pnas.2221542120
Application of a quantitative framework to improve the accuracy of a bacterial infection model
Abstract
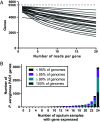
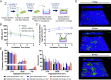
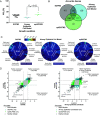
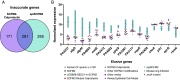
Laboratory models are critical to basic and translational microbiology research. Models serve multiple purposes, from providing tractable systems to study cell biology to allowing the investigation of inaccessible clinical and environmental ecosystems. Although there is a recognized need for improved model systems, there is a gap in rational approaches to accomplish this goal. We recently developed a framework for assessing the accuracy of microbial models by quantifying how closely each gene is expressed in the natural environment and in various models. The accuracy of the model is defined as the percentage of genes that are similarly expressed in the natural environment and the model. Here, we leverage this framework to develop and validate two generalizable approaches for improving model accuracy, and as proof of concept, we apply these approaches to improve models of Pseudomonas aeruginosa infecting the cystic fibrosis (CF) lung. First, we identify two models, an in vitro synthetic CF sputum medium model (SCFM2) and an epithelial cell model, that accurately recapitulate different gene sets. By combining these models, we developed the epithelial cell-SCFM2 model which improves the accuracy of over 500 genes. Second, to improve the accuracy of specific genes, we mined publicly available transcriptome data, which identified zinc limitation as a cue present in the CF lung and absent in SCFM2. Induction of zinc limitation in SCFM2 resulted in accurate expression of 90% of P. aeruginosa genes. These approaches provide generalizable, quantitative frameworks for microbiological model improvement that can be applied to any system of interest.
Keywords: Pseudomonas aeruginosa; calprotectin; cystic fibrosis; epithelial cell model; preclinical model.
Conflict of interest statement
The authors declare no competing interest.
Figures

References
-
- Hanley J. G., Models and microbiology: Pasteur and the body. Can Bull Med. Hist 20, 419–435 (2003). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

