Modeling human skeletal development using human pluripotent stem cells
- PMID: 37126720
- PMCID: PMC10175848
- DOI: 10.1073/pnas.2211510120
Modeling human skeletal development using human pluripotent stem cells
Abstract
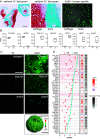
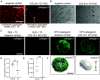
Chondrocytes and osteoblasts differentiated from induced pluripotent stem cells (iPSCs) will provide insights into skeletal development and genetic skeletal disorders and will generate cells for regenerative medicine applications. Here, we describe a method that directs iPSC-derived sclerotome to chondroprogenitors in 3D pellet culture then to articular chondrocytes or, alternatively, along the growth plate cartilage pathway to become hypertrophic chondrocytes that can transition to osteoblasts. Osteogenic organoids deposit and mineralize a collagen I extracellular matrix (ECM), mirroring in vivo endochondral bone formation. We have identified gene expression signatures at key developmental stages including chondrocyte maturation, hypertrophy, and transition to osteoblasts and show that this system can be used to model genetic cartilage and bone disorders.
Keywords: bone; cartilage; genetic skeletal disorder; iPSC.
Conflict of interest statement
The authors declare no competing interest.
Figures


References
-
- Karsenty G., Kronenberg H. M., Settembre C., Genetic control of bone formation. Annu. Rev. Cell Dev. Biol. 25, 629–648 (2009). - PubMed
-
- Kronenberg H. M., Developmental regulation of the growth plate. Nature 423, 332–336 (2003). - PubMed
-
- Tsang K. Y., Tsang S. W., Chan D., Cheah K. S. E., The chondrocytic journey in endochondral bone growth and skeletal dysplasia. Birth Defects Res. C Embryo Today 102, 52–73 (2014). - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

