PGE2 alters chromatin through H2A.Z-variant enhancer nucleosome modification to promote hematopoietic stem cell fate
- PMID: 37126722
- PMCID: PMC10175842
- DOI: 10.1073/pnas.2220613120
PGE2 alters chromatin through H2A.Z-variant enhancer nucleosome modification to promote hematopoietic stem cell fate
Abstract
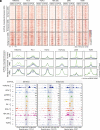
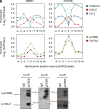
Prostaglandin E2 (PGE2) and 16,16-dimethyl-PGE2 (dmPGE2) are important regulators of hematopoietic stem and progenitor cell (HSPC) fate and offer potential to enhance stem cell therapies [C. Cutler et al. Blood 122, 3074-3081(2013); W. Goessling et al. Cell Stem Cell 8, 445-458 (2011); W. Goessling et al. Cell 136, 1136-1147 (2009)]. Here, we report that PGE2-induced changes in chromatin at enhancer regions through histone-variant H2A.Z permit acute inflammatory gene induction to promote HSPC fate. We found that dmPGE2-inducible enhancers retain MNase-accessible, H2A.Z-variant nucleosomes permissive of CREB transcription factor (TF) binding. CREB binding to enhancer nucleosomes following dmPGE2 stimulation is concomitant with deposition of histone acetyltransferases p300 and Tip60 on chromatin. Subsequent H2A.Z acetylation improves chromatin accessibility at stimuli-responsive enhancers. Our findings support a model where histone-variant nucleosomes retained within inducible enhancers facilitate TF binding. Histone-variant acetylation by TF-associated nucleosome remodelers creates the accessible nucleosome landscape required for immediate enhancer activation and gene induction. Our work provides a mechanism through which inflammatory mediators, such as dmPGE2, lead to acute transcriptional changes and modify HSPC behavior to improve stem cell transplantation.
Keywords: chromatin; hematopoietic stem cell; prostaglandin.
Conflict of interest statement
The authors have organizational affiliations to disclose, L.I.Z. is founder and stockholder of Fate, Inc., Scholar Rock, Camp4 Therapeutics, and Amagma Therapeutics and a scientific advisor for Stemgent. The other authors declare no competing interests.
Figures
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

