ESGO/ESTRO/ESP Guidelines for the management of patients with cervical cancer - Update 2023
- PMID: 37127326
- PMCID: PMC10176411
- DOI: 10.1136/ijgc-2023-004429
ESGO/ESTRO/ESP Guidelines for the management of patients with cervical cancer - Update 2023
Abstract
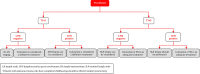
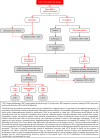
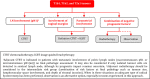
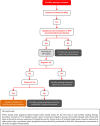
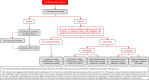
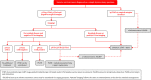
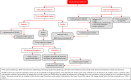
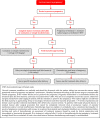
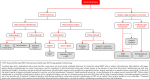
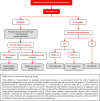
In 2018, the European Society of Gynecological Oncology (ESGO) jointly with the European Society for Radiotherapy and Oncology (ESTRO) and the European Society of Pathology (ESP) published evidence-based guidelines for the management of patients with cervical cancer. Given the large body of new evidence addressing the management of cervical cancer, the three sister societies jointly decided to update these evidence-based guidelines. The update includes new topics to provide comprehensive guidelines on all relevant issues of diagnosis and treatment in cervical cancer.To serve on the expert panel (27 experts across Europe) ESGO/ESTRO/ESP nominated practicing clinicians who are involved in managing patients with cervical cancer and have demonstrated leadership through their expertise in clinical care and research, national and international engagement, profile, and dedication to the topics addressed. To ensure the statements were evidence based, new data identified from a systematic search was reviewed and critically appraised. In the absence of any clear scientific evidence, judgment was based on the professional experience and consensus of the international development group. Before publication, the guidelines were reviewed by 155 independent international practitioners in cancer care delivery and patient representatives.These updated guidelines are comprehensive and cover staging, management, follow-up, long-term survivorship, quality of life and palliative care. Management includes fertility sparing treatment, early and locally advanced cervical cancer, invasive cervical cancer diagnosed on a simple hysterectomy specimen, cervical cancer in pregnancy, rare tumors, recurrent and metastatic diseases. The management algorithms and the principles of radiotherapy and pathological evaluation are also defined.
Keywords: Cervical Cancer; Pathology; Radiation; Surgical Oncology.
© 2023 IGCS and ESGO. This is an open access article under the CC BY license. Published by BMJ.
Conflict of interest statement
Competing interests: CCh has reported advisory boards for GSK, MSD and EISAI; SFL has reported advisory boards for MSD, GSK, AstraZeneca and Novartis; DL has reported consultant honoria from AstraZeneca, Clovis Oncology, GSK, MSD, Immunogen, Genmab, Amgen, Seagen and PharmaMar, advisory boards for AstraZeneca, Merck Serono, Seagen, Immunogen, Genmab, Oncoinvest, Corcept and Sutro, research institutional funding from Clovis Oncology, GSK, MSD and PharmaMar, research sponsored by AstraZeneca, Clovis Oncology, Genmab, GSK, Immunogen, Incyte, MSD, Roche, Seagen and Novartis, and speakers’ bureau activities for AstraZeneca, Clovis Oncology, GSK, MSD and PharmaMar; UM has reported advisory boards for AstraZeneca (Steering committee member for CALLA Study); RN has reported research grants from Elekta, Varian, Accuray, Dutch Research Council, and Dutch Cancer Society; AO has reported personal fees for advisory board membersip from Agenus, AstraZeneca, Clovis Oncology, Corcept Therapeutics, Deciphera Pharmaceuticals, Eisai, EMD Serono, F. Hoffmann-La Roche, Genmab/Seagen, GSK, ImmunoGen, Itheos, Merck Sharp & Dohme de Espana, SA, Mersana Thereapeutics, Novocure, PharmaMar, piIME Oncology, Roche, Sattucklabs, Sutro Biopharma and Tesaro, and personal fees for travel/accomodation from AstraZeneca, PharmaMar and Roche; DQ has reported advisory boards for Mimark inc; MPS has reported research grants and personal fees for workshops from Elekta AB; DC, MRR, FP, CC, AF, DF, DJK, FJ, CK, PM, RN, FPec, JP, SR, AS, VS, KT, IZ and JCL have reported no conflicts of interest.
Figures
Comment in
-
ESGO/ESTRO/ESP updated guidelines in cervical cancer.Int J Gynecol Cancer. 2023 May 1;33(5):667-668. doi: 10.1136/ijgc-2023-004523. Int J Gynecol Cancer. 2023. PMID: 37055170 No abstract available.
-
Correspondence on 'ESGO/ESTRO/ESP Guidelines for the management of patients with cervical cancer - Update 2023*'.Int J Gynecol Cancer. 2023 Jul 3;33(7):1165-1166. doi: 10.1136/ijgc-2023-004656. Int J Gynecol Cancer. 2023. PMID: 37295819 No abstract available.
-
Correspondence on "ESGO/ESTRO/ESP guidelines for the management of patients with cervical cancer: update 2023" by Cibula et al.Int J Gynecol Cancer. 2023 Jul 3;33(7):1167. doi: 10.1136/ijgc-2023-004645. Int J Gynecol Cancer. 2023. PMID: 37321672 No abstract available.
References
-
- Cibula D, Pötter R, Planchamp F, et al. The European Society of Gynaecological Oncology/European Society for Radiotherapy and Oncology/European Society of Pathology Guidelines for the management of patients with cervical cancer. Int J Gynecol Cancer 2018;28:641–55. 10.1097/IGC.0000000000001216 - DOI - PubMed
-
- Cibula D, Pötter R, Planchamp F, et al. The European Society of Gynaecological Oncology/European Society for Radiotherapy and Oncology/European Society of Pathology Guidelines for the management of patients with cervical cancer. Radiother Oncol 2018;127:404–16. 10.1016/j.radonc.2018.03.003 - DOI - PubMed