Structural Preservation Does Not Ensure Function at Sensory Ia-Motoneuron Synapses following Peripheral Nerve Injury and Repair
- PMID: 37127364
- PMCID: PMC10278679
- DOI: 10.1523/JNEUROSCI.0103-23.2023
Structural Preservation Does Not Ensure Function at Sensory Ia-Motoneuron Synapses following Peripheral Nerve Injury and Repair
Abstract
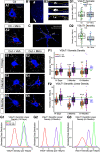
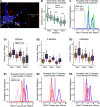
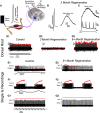
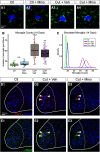
Injury that severs peripheral nerves often results in long-lasting motor behavioral deficits and in reorganization of related spinal motor circuitry, neither of which reverse even after nerve regeneration. Stretch areflexia and gait ataxia, for example, emerge from a combination of factors including degeneration of Ia-motoneuron synapses between peripherally damaged Ia muscle spindle afferents and motoneurons. Based on evidence that nerve injury acts via immune responses to induce synapse degeneration, we hypothesized that suppressing inflammatory responses would preserve Ia-motoneuron connectivity and aid in restoring normal function. We tested our hypothesis by administering the anti-inflammatory agent minocycline in male and female rats following axotomy of a peripheral nerve. The connectivity of Ia-motoneuron synapses was then assessed both structurally and functionally at different time points. We found that minocycline treatment overcame the physical loss of Ia contacts on motoneurons which are otherwise lost after axotomy. While necessary for functional recovery, synaptic preservation was not sufficient to overcome functional decline expressed as smaller than normal stretch-evoked synaptic potentials evoked monosynaptically at Ia-motoneuron connections and an absence of the stretch reflex. These findings demonstrate a limited capacity of minocycline to rescue normal sensorimotor behavior, illustrating that structural preservation of synaptic connectivity does not ensure normal synaptic function.SIGNIFICANCE STATEMENT Here we demonstrate that acute treatment with the semisynthetic tetracycline anti-inflammatory agent minocycline permanently prevents the comprehensive loss of synaptic contacts made between sensory neurons and spinal motoneurons following peripheral nerve injury and eventual regeneration. Treatment failed, however, to rescue normal function of those synapses or the reflex circuit they mediate. These findings demonstrate that preventing synaptic disconnection alone is not sufficient to restore neural circuit operation and associated sensorimotor behaviors.
Keywords: Ia afferents; degeneration; motoneurons; peripheral nerve injury; sensorimotor.
Copyright © 2023 the authors.
Figures


References
-
- Alvarez FJ, Titus-Mitchell HE, Bullinger KL, Kraszpulski M, Nardelli P, Cope TC (2011) Permanent central synaptic disconnection of proprioceptors after nerve injury and regeneration: I. Loss of VGLUT1/IA synapses on motoneurons. J Neurophysiol 106:2450–2470. 10.1152/jn.01095.2010 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
