Plasmodium falciparum gametocyte carriage in longitudinally monitored incident infections is associated with duration of infection and human host factors
- PMID: 37127688
- PMCID: PMC10150352
- DOI: 10.1038/s41598-023-33657-3
Plasmodium falciparum gametocyte carriage in longitudinally monitored incident infections is associated with duration of infection and human host factors
Abstract
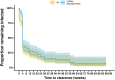
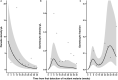
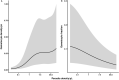
Malaria transmission depends on the presence of Plasmodium gametocytes that are the only parasite life stage that can infect mosquitoes. Gametocyte production varies between infections and over the course of infections. Infection duration is highly important for gametocyte production but poorly quantified. Between 2017 and 2019 an all-age cohort of individuals from Tororo, eastern Uganda was followed by continuous passive and routine assessments. We longitudinally monitored 104 incident infections from 98 individuals who were sampled once every 28 days and on any day of symptoms. Among infections that lasted ≥ 3 months, gametocyte appearance was near-universal with 96% of infections having detectable gametocytes prior to clearance. However, most infections were of much shorter duration; 55.7% of asymptomatic infections were detected only once. When considering all asymptomatic infections, regardless of their duration, only 36.3% had detectable gametocytes on at least one time-point prior to parasite clearance. Infections in individuals with sickle-cell trait (HbAS) were more likely to have gametocytes detected (Hazard Rate (HR) = 2.68, 95% CI 1.12, 6.38; p = 0.0231) and had gametocytes detected at higher densities (Density Ratio (DR) = 9.19, 95% CI 2.79, 30.23; p = 0.0002) compared to infections in wildtype (HbAA) individuals. Our findings suggest that a large proportion of incident infections is too short in duration and of too low density to contribute to onward transmission.
© 2023. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures



References
-
- WHO. World malaria report 2021 (Geneva, 2021).

