A rapid and flexible microneutralization assay for serological assessment of influenza viruses
- PMID: 37127782
- PMCID: PMC10174083
- DOI: 10.1111/irv.13141
A rapid and flexible microneutralization assay for serological assessment of influenza viruses
Abstract
Background: Serological responses from influenza vaccination or infection are typically measured by hemagglutinin inhibition (HAI) or microneutralization (MN). Both methods are limited in feasibility, standardization, and generalizability to recent strains. We developed a luciferase MN (LMN) assay that combines the advantages of the conventional MN assay with the ease of the HAI assay.
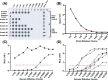
Methods: Sera were obtained from the HIVE study, a Michigan household cohort. Reverse genetics was used to generate recombinant influenza viruses expressing the hemagglutinin and neuraminidase of test strains, all other viral proteins from an A/WSN/1933 backbone, and a NanoLuc reporter. Serum neutralization of luciferase-expressing targets was quantified as a reduction in light emission from infected cells. Neutralization titers were measured for cell- and egg-adapted versions of A/Hong Kong/4801/2014 and A/Singapore/INFIMH-16-0019/2016 and compared to HAI titers against egg-grown antigens.
Results: Three hundred thirty-three sera were collected from 259 participants between May 2016 and July 2018. Sampled participants were 7-68 years of age, and >80% were vaccinated against influenza. HAI and LMN titers were correlated for A/Hong Kong/4801/2014 (ρ = 0.52, p ≤ 0.01) and A/Singapore/INFIMH-16-0019/2016 (ρ = 0.79, p ≤ 0.01). LMN titers were lower for cell strains compared to egg strains (A/Hong Kong/4801/2014 mean log2 fold change = -2.66, p ≤ 0.01 and A/Singapore/INFIMH-16-0019/2016 mean log2 fold change = -3.15, p ≤ 0.01).
Conclusions: The LMN assay was feasible using limited sample volumes and able to differentiate small antigenic differences between egg-adapted and cell-derived strains. The correspondence of these results with the commonly used HAI confirms the utility of this assay for high-throughput studies of correlates of protection and vaccine response.
Keywords: hemagglutination; influenza virus; neutralization; serology.
© 2023 The Authors. Influenza and Other Respiratory Viruses published by John Wiley & Sons Ltd.
Conflict of interest statement
Emily Martin has received research funding from Merck, unrelated to the submitted work. Arnold Monto and Adam Lauring have received consulting fees from Roche, unrelated to the submitted work.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

