Structural basis for RNA slicing by a plant Argonaute
- PMID: 37127820
- PMCID: PMC10868596
- DOI: 10.1038/s41594-023-00989-7
Structural basis for RNA slicing by a plant Argonaute
Abstract
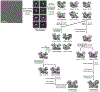
Argonaute (AGO) proteins use small RNAs to recognize transcripts targeted for silencing in plants and animals. Many AGOs cleave target RNAs using an endoribonuclease activity termed 'slicing'. Slicing by DNA-guided prokaryotic AGOs has been studied in detail, but structural insights into RNA-guided slicing by eukaryotic AGOs are lacking. Here we present cryogenic electron microscopy structures of the Arabidopsis thaliana Argonaute10 (AtAgo10)-guide RNA complex with and without a target RNA representing a slicing substrate. The AtAgo10-guide-target complex adopts slicing-competent and slicing-incompetent conformations that are unlike known prokaryotic AGO structures. AtAgo10 slicing activity is licensed by docking target (t) nucleotides t9-t13 into a surface channel containing the AGO endoribonuclease active site. A β-hairpin in the L1 domain secures the t9-t13 segment and coordinates t9-t13 docking with extended guide-target pairing. Results show that prokaryotic and eukaryotic AGOs use distinct mechanisms for achieving target slicing and provide insights into small interfering RNA potency.
© 2023. The Author(s), under exclusive licence to Springer Nature America, Inc.
Figures
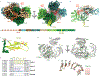

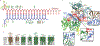







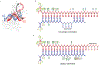


References
Methods-only references
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

