Retrospective examination of pseudoprogression in IDH mutant gliomas
- PMID: 37128507
- PMCID: PMC10148681
- DOI: 10.1093/noajnl/vdad028
Retrospective examination of pseudoprogression in IDH mutant gliomas
Abstract
Background: Tumor surveillance of isocitrate dehydrogenase (IDH) mutant gliomas is accomplished via serial contrast MRI. When new contrast enhancement (CEnew) is detected during postsurgical surveillance, clinicians must assess whether CEnew indicates pseudoprogression (PsP) or tumor progression (TP). PsP has been better studied in IDH wild-type glioblastoma but has not been well characterized in IDH mutant gliomas. We conducted a retrospective study evaluating the incidence, predictors, natural history, and survival of PsP patients in a large cohort of IDH mutant glioma patients treated at a single institution.
Methods: We identified 587 IDH mutant glioma patients treated at UCLA. We directly inspected MRI images and radiology reports to identify CEnew and categorized CEnew into TP or PsP using MRI or histopathology.
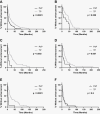
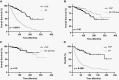
Results: Fifty-six percent of patients developed CEnew (326/587); of these, 92/326 patients (28% of CEnew; 16% of all) developed PsP and 179/326 (55%) developed TP. All PsP patients had prior radiation, chemotherapy, or chemoradiotherapy. PsP was associated with longer overall survival (OS) versus TP patients and similar OS versus no CEnew. PsP differs from TP based on earlier time of onset (median 5.8 vs 17.4 months from treatment, P < .0001) and MRI features that include punctate enhancement and enhancement location.
Conclusion: PsP patients represented 28% of CEnew patients and 16% of all patients; PsP patients demonstrated superior outcomes to TP patients, and equivalent survival to patients without CEnew. PsP persists for <1 year, occurs after treatment, and differs from TP based on time of onset and radiographic features. Poor outcomes after CEnew are driven by TP.
Keywords: IDH1/2; contrast enhancement; glioma; pseudoprogression; radiation necrosis.
© The Author(s) 2023. Published by Oxford University Press, the Society for Neuro-Oncology and the European Association of Neuro-Oncology.
Figures


References
-
- Stupp R, Mason WP, van den Bent MJ, et al. . Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. NEJM. 2005; 352(10): 987–996. Accessed September 28, 2022. https://www.nejm.org/doi/full/10.1056/NEJMoa043330 - DOI - PubMed
-
- Ellingson BM, Wen PY, Cloughesy TF.. Evidence and context of use for contrast enhancement as a surrogate of disease burden and treatment response in malignant glioma Neurooncol | Oxford Academic. 2017; 20(4): 457–471. Accessed May 24, 2022. https://academic.oup.com/neuro-oncology/article/20/4/457/4485469?login=true - PMC - PubMed
-
- Hempel JM, Brendle C, Bender B, et al. . Contrast enhancement predicting survival in integrated molecular subtypes of diffuse glioma: an observational cohort study. J Neurooncol. 2018;139(2):373–381. - PubMed
-
- Kruser TJ, Mehta MP, Robins HI.. Pseudoprogression after glioma therapy: a comprehensive review. Expert Rev Neurother. 2013;13(4):389–403. - PubMed
-
- Galldiks N, Kocher M, Langen KJ.. Pseudoprogression after glioma therapy: an update. Expert Rev Neurother. 2017;17(11):1109–1115. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
