Roles of host proteases in the entry of SARS-CoV-2
- PMID: 37128508
- PMCID: PMC10125864
- DOI: 10.1186/s44149-023-00075-x
Roles of host proteases in the entry of SARS-CoV-2
Abstract
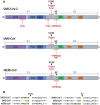
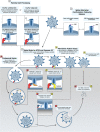
The spike protein (S) of SARS-CoV-2 is responsible for viral attachment and entry, thus a major factor for host susceptibility, tissue tropism, virulence and pathogenicity. The S is divided with S1 and S2 region, and the S1 contains the receptor-binding domain (RBD), while the S2 contains the hydrophobic fusion domain for the entry into the host cell. Numerous host proteases have been implicated in the activation of SARS-CoV-2 S through various cleavage sites. In this article, we review host proteases including furin, trypsin, transmembrane protease serine 2 (TMPRSS2) and cathepsins in the activation of SARS-CoV-2 S. Many betacoronaviruses including SARS-CoV-2 have polybasic residues at the S1/S2 site which is subjected to the cleavage by furin. The S1/S2 cleavage facilitates more assessable RBD to the receptor ACE2, and the binding triggers further conformational changes and exposure of the S2' site to proteases such as type II transmembrane serine proteases (TTPRs) including TMPRSS2. In the presence of TMPRSS2 on the target cells, SARS-CoV-2 can utilize a direct entry route by fusion of the viral envelope to the cellular membrane. In the absence of TMPRSS2, SARS-CoV-2 enter target cells via endosomes where multiple cathepsins cleave the S for the successful entry. Additional host proteases involved in the cleavage of the S were discussed. This article also includes roles of 3C-like protease inhibitors which have inhibitory activity against cathepsin L in the entry of SARS-CoV-2, and discussed the dual roles of such inhibitors in virus replication.
© The Author(s) 2023.
Conflict of interest statement
Competing interestsThe authors declare that they have no competing interests.
Figures

Similar articles
-
Distinctive Roles of Furin and TMPRSS2 in SARS-CoV-2 Infectivity.J Virol. 2022 Apr 27;96(8):e0012822. doi: 10.1128/jvi.00128-22. Epub 2022 Mar 28. J Virol. 2022. PMID: 35343766 Free PMC article.
-
Genome-wide bioinformatics analysis of human protease capacity for proteolytic cleavage of the SARS-CoV-2 spike glycoprotein.Microbiol Spectr. 2024 Feb 6;12(2):e0353023. doi: 10.1128/spectrum.03530-23. Epub 2024 Jan 8. Microbiol Spectr. 2024. PMID: 38189333 Free PMC article.
-
SARS-CoV-2 Spike Furin Cleavage Site and S2' Basic Residues Modulate the Entry Process in a Host Cell-Dependent Manner.J Virol. 2022 Jul 13;96(13):e0047422. doi: 10.1128/jvi.00474-22. Epub 2022 Jun 9. J Virol. 2022. PMID: 35678602 Free PMC article.
-
Proteolytic activation of SARS-CoV-2 spike protein.Microbiol Immunol. 2022 Jan;66(1):15-23. doi: 10.1111/1348-0421.12945. Epub 2021 Oct 12. Microbiol Immunol. 2022. PMID: 34561887 Free PMC article. Review.
-
SARS-CoV-2, Early Entry Events.J Pathog. 2020 Nov 24;2020:9238696. doi: 10.1155/2020/9238696. eCollection 2020. J Pathog. 2020. PMID: 33299610 Free PMC article. Review.
Cited by
-
Inhibitor design for TMPRSS2: insights from computational analysis of its backbone hydrogen bonds using a simple descriptor.Eur Biophys J. 2024 Feb;53(1-2):27-46. doi: 10.1007/s00249-023-01695-4. Epub 2023 Dec 29. Eur Biophys J. 2024. PMID: 38157015 Free PMC article.
-
Dandelion root extracts and taraxasterol inhibit LPS‑induced colorectal cancer cell viability by blocking TLR4‑NFκB‑driven ACE2 and TMPRSS2 pathways.Exp Ther Med. 2024 Apr 19;27(6):256. doi: 10.3892/etm.2024.12544. eCollection 2024 Jun. Exp Ther Med. 2024. PMID: 38766306 Free PMC article.
-
Aprotinin (II): Inhalational Administration for the Treatment of COVID-19 and Other Viral Conditions.Int J Mol Sci. 2024 Jun 29;25(13):7209. doi: 10.3390/ijms25137209. Int J Mol Sci. 2024. PMID: 39000315 Free PMC article. Review.
-
Association of Renin-Angiotensin Pathway Gene Polymorphisms with COVID-19 Susceptibility and Severity in Moroccans: A Case-Control Study.Biochem Genet. 2025 Jun;63(3):2225-2243. doi: 10.1007/s10528-024-10813-6. Epub 2024 May 8. Biochem Genet. 2025. PMID: 38717614
-
Aprotinin (I): Understanding the Role of Host Proteases in COVID-19 and the Importance of Pharmacologically Regulating Their Function.Int J Mol Sci. 2024 Jul 10;25(14):7553. doi: 10.3390/ijms25147553. Int J Mol Sci. 2024. PMID: 39062796 Free PMC article. Review.
References
-
- Abdelnabi R, Foo CS, Jochmans D, Vangeel L, De Jonghe S, Augustijns P, Mols R, Weynand B, Wattanakul T, Hoglund RM, Tarning J, Mowbray CE, Sjo P, Escudie F, Scandale I, Chatelain E, Neyts J. The oral protease inhibitor (PF-07321332) protects Syrian hamsters against infection with SARS-CoV-2 variants of concern. Nature Communications. 2022;13:719. doi: 10.1101/2021.11.04.467077. - DOI - PMC - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
