Visual processing in the fly, from photoreceptors to behavior
- PMID: 37128740
- PMCID: PMC10213501
- DOI: 10.1093/genetics/iyad064
Visual processing in the fly, from photoreceptors to behavior
Abstract
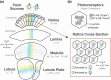
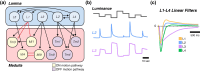
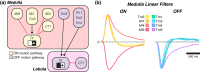
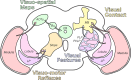
Originally a genetic model organism, the experimental use of Drosophila melanogaster has grown to include quantitative behavioral analyses, sophisticated perturbations of neuronal function, and detailed sensory physiology. A highlight of these developments can be seen in the context of vision, where pioneering studies have uncovered fundamental and generalizable principles of sensory processing. Here we begin with an overview of vision-guided behaviors and common methods for probing visual circuits. We then outline the anatomy and physiology of brain regions involved in visual processing, beginning at the sensory periphery and ending with descending motor control. Areas of focus include contrast and motion detection in the optic lobe, circuits for visual feature selectivity, computations in support of spatial navigation, and contextual associative learning. Finally, we look to the future of fly visual neuroscience and discuss promising topics for further study.
Keywords: FlyBook; anatomy; behavior; computation; learning; navigation; neuroscience; physiology; vision.
© The Author(s) 2023. Published by Oxford University Press on behalf of The Genetics Society of America.
Conflict of interest statement
Conflicts of interest The author(s) declare no conflict of interest.
Figures


References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

