Heterogeneity of Repolarization and Cell-Cell Variability of Cardiomyocyte Remodeling Within the Myocardial Infarction Border Zone Contribute to Arrhythmia Susceptibility
- PMID: 37128895
- PMCID: PMC10187631
- DOI: 10.1161/CIRCEP.122.011677
Heterogeneity of Repolarization and Cell-Cell Variability of Cardiomyocyte Remodeling Within the Myocardial Infarction Border Zone Contribute to Arrhythmia Susceptibility
Abstract
Background: After myocardial infarction, the infarct border zone (BZ) is the dominant source of life-threatening arrhythmias, where fibrosis and abnormal repolarization create a substrate for reentry. We examined whether repolarization abnormalities are heterogeneous within the BZ in vivo and could be related to heterogeneous cardiomyocyte remodeling.
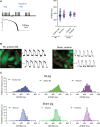
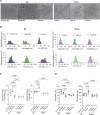
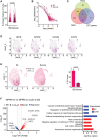
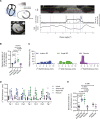
Methods: Myocardial infarction was induced in domestic pigs by 120-minute ischemia followed by reperfusion. After 1 month, remodeling was assessed by magnetic resonance imaging, and electroanatomical mapping was performed to determine the spatial distribution of activation-recovery intervals. Cardiomyocytes were isolated and tissue samples collected from the BZ and remote regions. Optical recording allowed assessment of action potential duration (di-8-ANEPPS, stimulation at 1 Hz, 37 °C) of large cardiomyocyte populations while gene expression in cardiomyocytes was determined by single nuclear RNA sequencing.
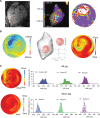
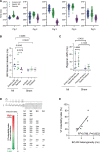
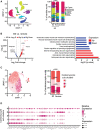
Results: In vivo, activation-recovery intervals in the BZ tended to be longer than in remote with increased spatial heterogeneity evidenced by a greater local SD (3.5±1.3 ms versus remote: 2.0±0.5 ms, P=0.036, npigs=5). Increased activation-recovery interval heterogeneity correlated with enhanced arrhythmia susceptibility. Cellular population studies (ncells=635-862 cells per region) demonstrated greater heterogeneity of action potential duration in the BZ (SD, 105.9±17.0 ms versus remote: 73.9±8.6 ms; P=0.001; npigs=6), which correlated with heterogeneity of activation-recovery interval in vivo. Cell-cell gene expression heterogeneity in the BZ was evidenced by increased Euclidean distances between nuclei of the BZ (12.1 [9.2-15.0] versus 10.6 [7.5-11.6] in remote; P<0.0001). Differentially expressed genes characterizing BZ cardiomyocyte remodeling included hypertrophy-related and ion channel-related genes with high cell-cell variability of expression. These gene expression changes were driven by stress-responsive TFs (transcription factors). In addition, heterogeneity of left ventricular wall thickness was greater in the BZ than in remote.
Conclusions: Heterogeneous cardiomyocyte remodeling in the BZ is driven by uniquely altered gene expression, related to heterogeneity in the local microenvironment, and translates to heterogeneous repolarization and arrhythmia vulnerability in vivo.
Keywords: arrhythmias; cardiac remodeling; hypertrophy; magnetic resonance imaging; myocardial infarction; single-cell gene expression analysis; voltage-sensitive dye imaging.
Conflict of interest statement
Figures

References
-
- Solomon SD, Zelenkofske S, McMurray JJV, Finn PV, Velazquez E, Ertl G, Harsanyi A, Rouleau JL, Maggioni A, Kober L, et al. ; Valsartan in Acute Myocardial Infarction Trial (VALIANT) Investigators. Sudden death in patients with myocardial infarction and left ventricular dysfunction, heart failure, or both. N Engl J Med. 2005;352:2581–2588. doi: 10.1056/NEJMoa043938 - PubMed
-
- Martin R, Maury P, Bisceglia C, Wong T, Estner H, Meyer C, Dallet C, Martin CA, Shi R, Takigawa M, et al. . Characteristics of scar-related ventricular tachycardia circuits using ultra-high-density mapping. Circ Arrhythm Electrophysiol. 2018;11:e006569. doi: 10.1161/CIRCEP.118.006569 - PubMed
-
- Nieves-Cintrón M, Hirenallur-Shanthappa D, Nygren PJ, Hinke SA, Dell’Acqua ML, Langeberg LK, Navedo M, Santana LF, Scott JD. AKAP150 participates in calcineurin/NFAT activation during the down-regulation of voltage-gated K+ currents in ventricular myocytes following myocardial infarction. Cell Signal. 2016;28:733–740. doi: 10.1016/j.cellsig.2015.12.015 - PMC - PubMed
-
- Rossow CF, Minami E, Chase EG, Murry CE, Santana LF. NFATc3-induced reductions in voltage-gated K+ currents after myocardial infarction. Circ Res. 2004;94:1340–1350. doi: 10.1161/01.RES.0000128406.08418.34 - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

