Restoration of Cardiac Myosin Light Chain Kinase Ameliorates Systolic Dysfunction by Reducing Superrelaxed Myosin
- PMID: 37128901
- PMCID: PMC10270284
- DOI: 10.1161/CIRCULATIONAHA.122.062885
Restoration of Cardiac Myosin Light Chain Kinase Ameliorates Systolic Dysfunction by Reducing Superrelaxed Myosin
Abstract
Background: Cardiac-specific myosin light chain kinase (cMLCK), encoded by MYLK3, regulates cardiac contractility through phosphorylation of ventricular myosin regulatory light chain. However, the pathophysiological and therapeutic implications of cMLCK in human heart failure remain unclear. We aimed to investigate whether cMLCK dysregulation causes cardiac dysfunction and whether the restoration of cMLCK could be a novel myotropic therapy for systolic heart failure.
Methods: We generated the knock-in mice (Mylk3+/fs and Mylk3fs/fs) with a familial dilated cardiomyopathy-associated MYLK3 frameshift mutation (MYLK3+/fs) that had been identified previously by us (c.1951-1G>T; p.P639Vfs*15) and the human induced pluripotent stem cell-derived cardiomyocytes from the carrier of the mutation. We also developed a new small-molecule activator of cMLCK (LEUO-1154).
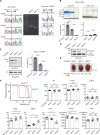
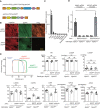
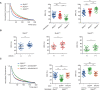
Results: Both mice (Mylk3+/fs and Mylk3fs/fs) showed reduced cMLCK expression due to nonsense-mediated messenger RNA decay, reduced MLC2v (ventricular myosin regulatory light chain) phosphorylation in the myocardium, and systolic dysfunction in a cMLCK dose-dependent manner. Consistent with this result, myocardium from the mutant mice showed an increased ratio of cardiac superrelaxation/disordered relaxation states that may contribute to impaired cardiac contractility. The phenotypes observed in the knock-in mice were rescued by cMLCK replenishment through the AAV9_MYLK3 vector. Human induced pluripotent stem cell-derived cardiomyocytes with MYLK3+/fs mutation reduced cMLCK expression by 50% and contractile dysfunction, accompanied by an increased superrelaxation/disordered relaxation ratio. CRISPR-mediated gene correction, or cMLCK replenishment by AAV9_MYLK3 vector, successfully recovered cMLCK expression, the superrelaxation/disordered relaxation ratio, and contractile dysfunction. LEUO-1154 increased human cMLCK activity ≈2-fold in the Vmax for ventricular myosin regulatory light chain phosphorylation without affecting the Km. LEUO-1154 treatment of human induced pluripotent stem cell-derived cardiomyocytes with MYLK3+/fs mutation restored the ventricular myosin regulatory light chain phosphorylation level and superrelaxation/disordered relaxation ratio and improved cardiac contractility without affecting calcium transients, indicating that the cMLCK activator acts as a myotrope. Finally, human myocardium from advanced heart failure with a wide variety of causes had a significantly lower MYLK3/PPP1R12B messenger RNA expression ratio than control hearts, suggesting an altered balance between myosin regulatory light chain kinase and phosphatase in the failing myocardium, irrespective of the causes.
Conclusions: cMLCK dysregulation contributes to the development of cardiac systolic dysfunction in humans. Our strategy to restore cMLCK activity could form the basis of a novel myotropic therapy for advanced systolic heart failure.
Keywords: cardiac myosin; left; myosin light chain kinase; phosphorylation; ventricular dysfunction.
Conflict of interest statement
Figures




Comment in
-
Letter by Okamoto et al Regarding Article, "Restoration of Cardiac Myosin Light Chain Kinase Ameliorates Systolic Dysfunction by Reducing Superrelaxed Myosin".Circulation. 2023 Dec 19;148(25):2073. doi: 10.1161/CIRCULATIONAHA.123.066090. Epub 2023 Dec 18. Circulation. 2023. PMID: 38109342 No abstract available.
-
Letter by Komamura Regarding Article "Restoration of Cardiac Myosin Light Chain Kinase Ameliorates Systolic Dysfunction by Reducing Superrelaxed Myosin".Circulation. 2023 Dec 19;148(25):2072. doi: 10.1161/CIRCULATIONAHA.123.066540. Epub 2023 Dec 18. Circulation. 2023. PMID: 38109344 No abstract available.
References
-
- Teerlink JR, Diaz R, Felker GM, McMurray JJV, Metra M, Solomon SD, Adams KF, Anand I, Arias-Mendoza A, Biering-Sorensen T, et al. ; GALACTIC-HF Investigators. Cardiac myosin activation with omecamtiv mecarbil in systolic heart failure. N Engl J Med. 2021;384:105–116. doi: 10.1056/NEJMoa2025797 - PubMed
-
- Psotka MA, Gottlieb SS, Francis GS, Allen LA, Teerlink JR, Adams KF, Jr, Rosano GMC, Lancellotti P. Cardiac calcitropes, myotropes, and mitotropes: JACC review topic of the week. J Am Coll Cardiol. 2019;73:2345–2353. doi: 10.1016/j.jacc.2019.02.051 - PubMed
-
- Felker GM, Solomon SD, Claggett B, Diaz R, McMurray JJV, Metra M, Anand I, Crespo-Leiro MG, Dahlstrom U, Goncalvesova E, et al. Assessment of omecamtiv mecarbil for the treatment of patients with severe heart failure: a post hoc analysis of data from the GALACTIC-HF randomized clinical trial. JAMA Cardiol. 2022;7:26–34. doi: 10.1001/jamacardio.2021.4027 - PMC - PubMed
-
- Hwang PM, Sykes BD. Targeting the sarcomere to correct muscle function. Nat Rev Drug Discovery. 2015;14:313–328. doi: 10.1038/nrd4554 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

