Identification via Virtual Screening of Emissive Molecules with a Small Exciton-Vibration Coupling for High Color Purity and Potential Large Exciton Delocalization
- PMID: 37129191
- PMCID: PMC10165648
- DOI: 10.1021/acs.jpclett.3c00749
Identification via Virtual Screening of Emissive Molecules with a Small Exciton-Vibration Coupling for High Color Purity and Potential Large Exciton Delocalization
Abstract
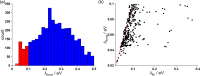
A sequence of quantum chemical computations of increasing accuracy was used in this work to identify molecules with small exciton reorganization energy (exciton-vibration coupling), of interest for light emitting devices and coherent exciton transport, starting from a set of ∼4500 known molecules. We validated an approximate computational approach based on single-point calculations of the force in the excited state, which was shown to be very efficient in identifying the most promising candidates. We showed that a simple descriptor based on the bond order could be used to find molecules with potentially small exciton reorganization energies without performing excited state calculations. A small set of chemically diverse molecules with a small exciton reorganization energy was analyzed in greater detail to identify common features leading to this property. Many such molecules display an A-B-A structure where the bonding/antibonding patterns in the fragments A are similar in HOMO and LUMO. Another group of molecules with small reorganization energy displays instead HOMO and LUMO with a strong nonbonding character.
Conflict of interest statement
The authors declare no competing financial interest.
Figures
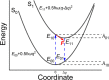
 and F = ℏωΔq. Equations in the manuscript are for potential energy
surfaces of higher dimensions.
and F = ℏωΔq. Equations in the manuscript are for potential energy
surfaces of higher dimensions.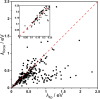



Similar articles
-
Efficient Near-Infrared Luminescence of Self-Assembled Platinum(II) Complexes: From Fundamentals to Applications.Acc Chem Res. 2023 Mar 21;56(6):689-699. doi: 10.1021/acs.accounts.2c00827. Epub 2023 Mar 7. Acc Chem Res. 2023. PMID: 36882976
-
Spectral shift, electronic coupling and exciton delocalization in nanocrystal dimers: insights from all-atom electronic structure computations.Nanoscale. 2020 Sep 17;12(35):18124-18136. doi: 10.1039/d0nr05601d. Nanoscale. 2020. PMID: 32852028
-
Tuning Hybridized Local and Charge-Transfer Mixing for Efficient Hot-Exciton Emission with Improved Color Purity.J Phys Chem Lett. 2022 Jul 28;13(29):6664-6673. doi: 10.1021/acs.jpclett.2c01917. Epub 2022 Jul 15. J Phys Chem Lett. 2022. PMID: 35839081
-
The properties of bio-energy transport and influence of structure nonuniformity and temperature of systems on energy transport along polypeptide chains.Prog Biophys Mol Biol. 2012 Jan;108(1-2):1-46. doi: 10.1016/j.pbiomolbio.2011.09.005. Epub 2011 Sep 17. Prog Biophys Mol Biol. 2012. PMID: 21951575 Review.
-
Intramolecular Vibrations in Excitation Energy Transfer: Insights from Real-Time Path Integral Calculations.Annu Rev Phys Chem. 2022 Apr 20;73:349-375. doi: 10.1146/annurev-physchem-090419-120202. Epub 2022 Jan 26. Annu Rev Phys Chem. 2022. PMID: 35081322 Review.
Cited by
-
Directed exciton transport highways in organic semiconductors.Nat Commun. 2023 Sep 12;14(1):5599. doi: 10.1038/s41467-023-41044-9. Nat Commun. 2023. PMID: 37699907 Free PMC article.
-
Exciton Transport in the Nonfullerene Acceptor O-IDTBR from Nonadiabatic Molecular Dynamics.J Chem Theory Comput. 2024 Jul 23;20(14):6241-6252. doi: 10.1021/acs.jctc.4c00605. Epub 2024 Jul 5. J Chem Theory Comput. 2024. PMID: 38967252 Free PMC article.
-
Calibration of several first excited state properties for organic molecules through systematic comparison of TDDFT with experimental spectra.J Mater Chem C Mater. 2024 Oct 14;12(46):18886-18892. doi: 10.1039/d4tc03511a. eCollection 2024 Nov 28. J Mater Chem C Mater. 2024. PMID: 39444434 Free PMC article.
References
-
- Geffroy B.; Le Roy P.; Prat C. Organic light-emitting diode (OLED) technology: materials, devices and display technologies. Polym. Int. 2006, 55 (6), 572–582. 10.1002/pi.1974. - DOI
-
- Jou J.-H.; Kumar S.; Agrawal A.; Li T.-H.; Sahoo S. Approaches for fabricating high efficiency organic light emitting diodes. J. Mater. Chem. C 2015, 3 (13), 2974–3002. 10.1039/C4TC02495H. - DOI
-
- Ahmad S. A.; Eng J.; Penfold T. J. Rapid predictions of the colour purity of luminescent organic molecules. J. Mater. Chem. C 2022, 10 (12), 4785–4794. 10.1039/D1TC04748E. - DOI
LinkOut - more resources
Full Text Sources

