Photodynamic and Photothermal Therapies: Synergy Opportunities for Nanomedicine
- PMID: 37129253
- PMCID: PMC10173698
- DOI: 10.1021/acsnano.3c00891
Photodynamic and Photothermal Therapies: Synergy Opportunities for Nanomedicine
Abstract
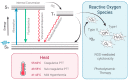
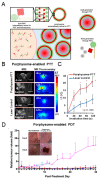
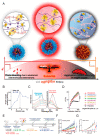
Tumoricidal photodynamic (PDT) and photothermal (PTT) therapies harness light to eliminate cancer cells with spatiotemporal precision by either generating reactive oxygen species or increasing temperature. Great strides have been made in understanding biological effects of PDT and PTT at the cellular, vascular and tumor microenvironmental levels, as well as translating both modalities in the clinic. Emerging evidence suggests that PDT and PTT may synergize due to their different mechanisms of action, and their nonoverlapping toxicity profiles make such combination potentially efficacious. Moreover, PDT/PTT combinations have gained momentum in recent years due to the development of multimodal nanoplatforms that simultaneously incorporate photodynamically- and photothermally active agents. In this review, we discuss how combining PDT and PTT can address the limitations of each modality alone and enhance treatment safety and efficacy. We provide an overview of recent literature featuring dual PDT/PTT nanoparticles and analyze the strengths and limitations of various nanoparticle design strategies. We also detail how treatment sequence and dose may affect cellular states, tumor pathophysiology and drug delivery, ultimately shaping the treatment response. Lastly, we analyze common experimental design pitfalls that complicate preclinical assessment of PDT/PTT combinations and propose rational guidelines to elucidate the mechanisms underlying PDT/PTT interactions.
Keywords: PDT; PTT; cancer; combination therapies; drug delivery; multimodal nanoparticles; photodynamic therapy; photomedicine; photothermal therapy; theranostics.
Conflict of interest statement
The authors declare no competing financial interest.
Figures




References
-
- Huang H. C.; Rizvi I.; Liu J.; Anbil S.; Kalra A.; Lee H.; Baglo Y.; Paz N.; Hayden D.; Pereira S.; Pogue B. W.; Fitzgerald J.; Hasan T. Photodynamic Priming Mitigates Chemotherapeutic Selection Pressures and Improves Drug Delivery. Cancer Res. 2018, 78 (2), 558–571. 10.1158/0008-5472.CAN-17-1700. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

