Mitochondrial protein import clogging as a mechanism of disease
- PMID: 37129366
- PMCID: PMC10208645
- DOI: 10.7554/eLife.84330
Mitochondrial protein import clogging as a mechanism of disease
Abstract
Mitochondrial biogenesis requires the import of >1,000 mitochondrial preproteins from the cytosol. Most studies on mitochondrial protein import are focused on the core import machinery. Whether and how the biophysical properties of substrate preproteins affect overall import efficiency is underexplored. Here, we show that protein traffic into mitochondria can be disrupted by amino acid substitutions in a single substrate preprotein. Pathogenic missense mutations in ADP/ATP translocase 1 (ANT1), and its yeast homolog ADP/ATP carrier 2 (Aac2), cause the protein to accumulate along the protein import pathway, thereby obstructing general protein translocation into mitochondria. This impairs mitochondrial respiration, cytosolic proteostasis, and cell viability independent of ANT1's nucleotide transport activity. The mutations act synergistically, as double mutant Aac2/ANT1 causes severe clogging primarily at the translocase of the outer membrane (TOM) complex. This confers extreme toxicity in yeast. In mice, expression of a super-clogger ANT1 variant led to neurodegeneration and an age-dependent dominant myopathy that phenocopy ANT1-induced human disease, suggesting clogging as a mechanism of disease. More broadly, this work implies the existence of uncharacterized amino acid requirements for mitochondrial carrier proteins to avoid clogging and subsequent disease.
Keywords: Ant1; S. cerevisiae; biochemistry; chemical biology; clogging; disease; mPOS; mitochondria; mouse; protein import.
Plain language summary
Inside our cells, compartments known as mitochondria generate the chemical energy required for life processes to unfold. Most of the proteins found within mitochondria are manufactured in another part of the cell (known as the cytosol) and then imported with the help of specialist machinery. For example, the TOM and TIM22 channels provide a route for the proteins to cross the two membrane barriers that separate the cytosol from the inside of a mitochondrion. ANT1 is a protein that is found inside mitochondria in humans, where it acts as a transport system for the cell’s energy currency. Specific mutations in the gene encoding ANT1 have been linked to degenerative conditions that affect the muscles and the brain. However, it remains unclear how these mutations cause disease. To address this question, Coyne et al. recreated some of the mutations in the gene encoding the yeast equivalent of ANT1 (known as Aac2). Experiments in yeast cells carrying these mutations showed that the Aac2 protein accumulated in the TOM and TIM22 channels, creating a ‘clog’ that prevented other essential proteins from reaching the mitochondria. As a result, the yeast cells died. Mutant forms of the human ANT1 protein also clogged up the TOM and TIM22 channels of human cells in a similar way. Further experiments focused on mice genetically engineered to produce a “super-clogger” version of the mouse equivalent of ANT1. The animals soon developed muscle and neurological conditions similar to those observed in human diseases associated with ANT1. The findings of Coyne et al. suggest that certain genetic mutations in the gene encoding the ANT1 protein cause disease by blocking the transport of other proteins to the mitochondria, rather than by directly affecting ANT1’s nucleotide trnsport role in the cell. This redefines our understanding of diseases associated with mitochondrial proteins, potentially altering how treatments for these conditions are designed.
© 2023, Coyne et al.
Conflict of interest statement
LC, XW, JS, Ed, KS, PM, FM, TB, XC No competing interests declared
Figures
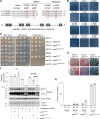
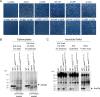
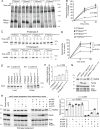
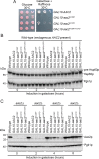











Update of
References
-
- Araiso Y, Tsutsumi A, Qiu J, Imai K, Shiota T, Song J, Lindau C, Wenz L-S, Sakaue H, Yunoki K, Kawano S, Suzuki J, Wischnewski M, Schütze C, Ariyama H, Ando T, Becker T, Lithgow T, Wiedemann N, Pfanner N, Kikkawa M, Endo T. Structure of the mitochondrial import gate reveals distinct preprotein paths. Nature. 2019;575:395–401. doi: 10.1038/s41586-019-1680-7. - DOI - PubMed
-
- Backes S, Bykov YS, Flohr T, Räschle M, Zhou J, Lenhard S, Krämer L, Mühlhaus T, Bibi C, Jann C, Smith JD, Steinmetz LM, Rapaport D, Storchová Z, Schuldiner M, Boos F, Herrmann JM. The chaperone-binding activity of the mitochondrial surface receptor Tom70 protects the cytosol against mitoprotein-induced stress. Cell Reports. 2021;35:108936. doi: 10.1016/j.celrep.2021.108936. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

