Helical Bilayer Nanographenes: Impact of the Helicene Length on the Structural, Electrochemical, Photophysical, and Chiroptical Properties
- PMID: 37129470
- PMCID: PMC10236438
- DOI: 10.1021/jacs.3c01088
Helical Bilayer Nanographenes: Impact of the Helicene Length on the Structural, Electrochemical, Photophysical, and Chiroptical Properties
Abstract
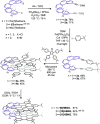
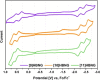
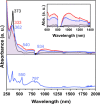
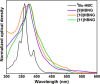
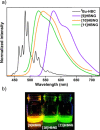
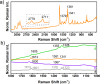
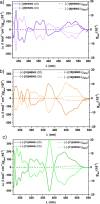
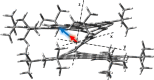
Helical bilayer nanographenes (HBNGs) are chiral π-extended aromatic compounds consisting of two π-π stacked hexabenzocoronenes (HBCs) joined by a helicene, thus resembling van der Waals layered 2D materials. Herein, we compare [9]HBNG, [10]HBNG, and [11]HBNG helical bilayers endowed with [9], [10], and [11]helicenes embedded in their structure, respectively. Interestingly, the helicene length defines the overlapping degree between the two HBCs (number of benzene rings involved in π-π interactions between the two layers), being 26, 14, and 10 benzene rings, respectively, according to the X-ray analysis. Unexpectedly, the electrochemical study shows that the lesser π-extended system [9]HBNG shows the strongest electron donor character, in part by interlayer exchange resonance, and more red-shifted values of emission. Furthermore, [9]HBNG also shows exceptional chiroptical properties with the biggest values of gabs and glum (3.6 × 10-2) when compared to [10]HBNG and [11]HBNG owing to the fine alignment in the configuration of [9]HBNG between its electric and magnetic dipole transition moments. Furthermore, spectroelectrochemical studies as well as the fluorescence spectroscopy support the aforementioned experimental findings, thus confirming the strong impact of the helicene length on the properties of this new family of bilayer nanographenes.
Conflict of interest statement
The authors declare no competing financial interest.
Figures



References
-
- Hu Y.; Wu C.; Pan Q.; Jin Y.; Lyu R.; Martínez V.; Huang S.; Wu J.; Wayment L. J.; Clark N. A.; Raschke M. B.; Zhao Y.; Zhang W. Synthesis of γ-graphyne using dynamic covalent chemistry. Nat. Synth. 2022, 1, 449–454. 10.1038/s44160-022-00068-7. - DOI
-
- Mannix A. J.; Zhou X. F.; Kiraly B.; Wood J. D.; Alducin D.; Myers B. D.; Liu X.; Fisher B. L.; Santiago U.; Guest J. R.; Yacaman M. J.; Ponce A.; Oganov A. R.; Hersam M. C.; Guisinger N. P. Synthesis of borophenes: Anisotropic, two-dimensional boron polymorphs. Science 2015, 350, 1513–1516. 10.1126/science.aad1080. - DOI - PMC - PubMed
-
- Dávila M. E.; Xian L.; Cahangirov S.; Rubio A.; Le Lay G. Germanene: a novel two-dimensional germanium allotrope akin to graphene and silicene. New J. Phys. 2014, 16, 095002.10.1088/1367-2630/16/9/095002. - DOI
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

