Cell and Tissue Tropism of Brucella spp
- PMID: 37129522
- PMCID: PMC10187126
- DOI: 10.1128/iai.00062-23
Cell and Tissue Tropism of Brucella spp
Abstract
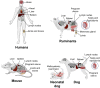
Brucella spp. are facultatively intracellular bacteria that can infect, survive, and multiply in various host cell types in vivo and/or in vitro. The genus Brucella has markedly expanded in recent years with the identification of novel species and hosts, which has revealed additional information about the cell and tissue tropism of these pathogens. Classically, Brucella spp. are considered to have tropism for organs that contain large populations of phagocytes such as lymph nodes, spleen, and liver, as well as for organs of the genital system, including the uterus, epididymis, testis, and placenta. However, experimental infections of several different cultured cell types indicate that Brucella may actually have a broader cell tropism than previously thought. Indeed, recent studies indicate that certain Brucella species in particular hosts may display a pantropic distribution in vivo. This review discusses the available knowledge on cell and tissue tropism of Brucella spp. in natural infections of various host species, as well as in experimental animal models and cultured cells.
Keywords: Brucella; macrophage; trophoblast; tropism.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
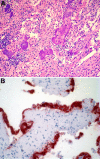
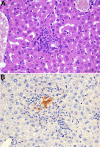

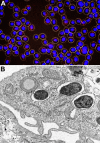
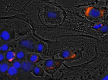

References
-
- Corbel MJ. 2006. Brucellosis in humans and animals. WHO Press, Geneva, Switzerland.
-
- Verger JM, Grimont F, Grimont PAD, Grayon M. 1985. Brucella, a monospecific genus as shown by deoxyribonucleic-acid hybridization. Int J Syst Evol Microbiol 35:292–295. doi:10.1099/00207713-35-3-292. - DOI
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

