BRCA2 Germline Mutations Identify Gastric Cancers Responsive to PARP Inhibitors
- PMID: 37129948
- PMCID: PMC10183806
- DOI: 10.1158/0008-5472.CAN-22-2620
BRCA2 Germline Mutations Identify Gastric Cancers Responsive to PARP Inhibitors
Abstract
Despite negative results of clinical trials conducted on the overall population of patients with gastric cancer, PARP inhibitor (PARPi) therapeutic strategy still might represent a window of opportunity for a subpopulation of patients with gastric cancer. An estimated 7% to 12% of gastric cancers exhibit a mutational signature associated with homologous recombination (HR) failure, suggesting that these patients could potentially benefit from PARPis. To analyze responsiveness of gastric cancer to PARPi, we exploited a gastroesophageal adenocarcinoma (GEA) platform of patient-derived xenografts (PDX) and PDX-derived primary cells and selected 10 PDXs with loss-of-function mutations in HR pathway genes. Cell viability assays and preclinical trials showed that olaparib treatment was effective in PDXs harboring BRCA2 germline mutations and somatic inactivation of the second allele. Olaparib responsive tumors were sensitive to oxaliplatin as well. Evaluation of HR deficiency (HRD) and mutational signatures efficiently stratified responder and nonresponder PDXs. A retrospective analysis on 57 patients with GEA showed that BRCA2 inactivating variants were associated with longer progression-free survival upon platinum-based regimens. Five of 7 patients with BRCA2 germline mutations carried the p.K3326* variant, classified as "benign." However, familial history of cancer, the absence of RAD51 foci in tumor cells, and a high HRD score suggest a deleterious effect of this mutation in gastric cancer. In conclusion, PARPis could represent an effective therapeutic option for BRCA2-mutated and/or high HRD score patients with GEA, including patients with familial intestinal gastric cancer.
Significance: PARP inhibition is a potential strategy for treating patients with gastric cancer with mutated BRCA2 or homologous repair deficiency, including patients with familial intestinal gastric cancer, for whom BRCA2 germline testing should be recommended.
©2023 The Authors; Published by the American Association for Cancer Research.
Figures
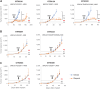
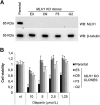
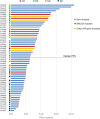
![Figure 1. GEA primary cells bearing BRCA2 germline mutations and loss of the WT allele are sensitive to PARPis. Boxplots showing the GR50 of primary cells derived from gastric cancer PDXs exposed to 3 different PARPis: olaparib, niraparib, and rucaparib. Boxes indicate the median ± standard deviation of GR50 values of 3 independent dose–response experiments (dots). GR50 and statistical significance (Wilcoxon rank-sum test) were calculated using the GRcalculator tool [(14); see Methods for details].](https://cdn.ncbi.nlm.nih.gov/pmc/blobs/ad33/10183806/4761ba5a3971/1699fig1.gif)


References
-
- Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018;68:394–424. - PubMed
-
- Bang YJ, Van Cutsem E, Feyereislova A, Chung HC, Shen L, Sawaki A, et al. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastroesophageal junction cancer (ToGA): a phase III, open-label, randomized controlled trial. Lancet 2010;376:687–97. - PubMed
-
- Wilke H, Muro K, Van Cutsem E, Oh SC, Bodoky G, Shimada Y, et al. Ramucirumab plus paclitaxel versus placebo plus paclitaxel in patients with previously treated advanced gastric or gastroesophageal junction adenocarcinoma (RAINBOW): a double-blind, randomized phase III trial. Lancet Oncol 2014;15:1224–35. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

