ACVIM consensus statement guidelines on diagnosing and distinguishing low-grade neoplastic from inflammatory lymphocytic chronic enteropathies in cats
- PMID: 37130034
- PMCID: PMC10229359
- DOI: 10.1111/jvim.16690
ACVIM consensus statement guidelines on diagnosing and distinguishing low-grade neoplastic from inflammatory lymphocytic chronic enteropathies in cats
Abstract
Background: Lymphoplasmacytic enteritis (LPE) and low-grade intestinal T cell lymphoma (LGITL) are common diseases in older cats, but their diagnosis and differentiation remain challenging.
Objectives: To summarize the current literature on etiopathogenesis and diagnosis of LPE and LGITL in cats and provide guidance on the differentiation between LPE and LGITL in cats. To provide statements established using evidence-based approaches or where such evidence is lacking, statements based on consensus of experts in the field.
Animals: None.
Methods: A panel of 6 experts in the field (2 internists, 1 radiologist, 1 anatomic pathologist, 1 clonality expert, 1 oncologist) with the support of a human medical immunologist, was formed to assess and summarize evidence in the peer-reviewed literature and complement it with consensus recommendations.
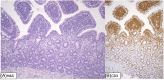
Results: Despite increasing interest on the topic for clinicians and pathologists, few prospective studies were available, and interpretation of the pertinent literature often was challenging because of the heterogeneity of the cases. Most recommendations by the panel were supported by a moderate or low level of evidence. Several understudied areas were identified, including cellular markers using immunohistochemistry, genomics, and transcriptomic studies.
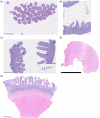
Conclusions and clinical importance: To date, no single diagnostic criterion or known biomarker reliably differentiates inflammatory lesions from neoplastic lymphoproliferations in the intestinal tract of cats and a diagnosis currently is established by integrating all available clinical and diagnostic data. Histopathology remains the mainstay to better differentiate LPE from LGITL in cats with chronic enteropathy.
Keywords: T-cell; alimentary; cat; chronic diarrhea; endoscopy; gastrointestinal; histology; immunohistochemistry; inflammatory bowel disease; lymphoma; lymphoplasmacytic enteritis; lymphoproliferative disorders.
© 2023 The Authors. Journal of Veterinary Internal Medicine published by Wiley Periodicals LLC on behalf of American College of Veterinary Internal Medicine.
Conflict of interest statement
Dr. S. Marsilio is a paid consultant for Dutch Pet, Inc., an online veterinary pet telehealth service and a paid speaker for Idexx Laboratories, Westbrook, ME.
Dr. V. Freiche is a paid speaker for Royal Canin, Aimargues, France, Dômes Pharma Vétérinaire, Lempdes, France, and Nestlé Purina, St Louis, MO.
Dr. E Johnson has nothing to disclose.
Dr. C. Leo is a paid consultant for Mars Anicura Inc., a paid teleconsultant for Vet‐CT, an online veterinary pet telehealth service based in the UK and a paid speaker for UNISVET, an Italy‐based continuing education company.
Dr. A.W. Langerak is the director of the Laboratory Medical Immunology (LMI) (ISO 15189 certified) at the Erasmus MC, University Medical Center, Rotterdam, The Netherlands. The LMI provides patient services including clonality testing on a fee‐for‐service basis. Dr. A.W. Langerak receives funding for research support from Roche‐Genentech, South San Francisco, CA, Janssen, Beerse, Belgium, and Gilead, Foster City, CA. Dr. A.W. Langerak is a paid speaker for Janssen, Beerse, Belgium, Gilead, Foster City, CA, and AbbVie, North Chicago, IL. Dr. A.W. Langerak is also a founding member of the EuroClonality/BIOMED‐2 group, a non‐profit organization providing analytical guidelines for the performance of clonality assays in human medicine.
Dr. I. Peters is an employee at the Veterinary Pathology Group (VPG), Exeter, Devon, UK which provides clonality testing and other laboratory services on a fee‐for‐service basis. None of these organizations influenced the outcome of this consensus statement.
Dr. M. Ackermann has nothing to disclose.
Figures






References
-
- Louwerens M, London CA, Pedersen NC, Lyons LA. Feline lymphoma in the post‐feline leukemia virus era. J Vet Intern Med. 2005;19:329‐335. - PubMed
-
- Freiche V, Cordonnier N, Paulin MV, et al. Feline low‐grade intestinal T cell lymphoma: a unique natural model of human indolent T cell lymphoproliferative disorder of the gastrointestinal tract. Lab Invest. 2021;101:794‐804. - PubMed
-
- Vaillant AAJ, Stang CM. Lymphoproliferative Disorders. Treasure Island, FL: StatPearls Publishing; 2021.
-
- Moticka EJ. A Historical Perspective on Evidence‐Based Immunology. Amsterdam, Netherlands: Elsevier; 2015.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Miscellaneous

