In situ assessment of Mindin as a biomarker of podocyte lesions in diabetic nephropathy
- PMID: 37130106
- PMCID: PMC10153717
- DOI: 10.1371/journal.pone.0284789
In situ assessment of Mindin as a biomarker of podocyte lesions in diabetic nephropathy
Abstract
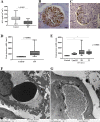
Diabetic nephropathy (DN) is the leading cause of chronic kidney disease and end-stage renal failure worldwide. Several mechanisms are involved in the pathogenesis of this disease, which culminate in morphological changes such as podocyte injury. Despite the complex diagnosis and pathogenesis, limited attempts have been made to establish new biomarkers for DN. The higher concentration of Mindin protein in the urine of patients with type 2 diabetes mellitus suggests that it plays a role in DN. Therefore, this study investigated whether in situ protein expression of Mindin can be considered a potential DN biomarker. Fifty renal biopsies from patients diagnosed with DN, 57 with nondiabetic glomerular diseases, including 17 with focal segmental glomerulosclerosis (FSGS), 14 with minimal lesion disease (MLD) and 27 with immunoglobulin A nephropathy (IgAN), and 23 adult kidney samples from autopsies (control group) were evaluated for Mindin expression by immunohistochemistry. Podocyte density was inferred by Wilms' tumor 1 (WT1) immunostaining, while foot process effacement was assessed by transmission electron microscopy. Receiver operative characteristic (ROC) analysis was performed to determine the biomarker sensitivity/specificity. Low podocyte density and increased Mindin expression were observed in all cases of DN, regardless of their class. In the DN group, Mindin expression was significantly higher than that in the FSGS, MCD, IgAN and control groups. Higher Mindin expression was significantly positively correlated with foot process effacement only in class III DN cases. Furthermore, Mindin protein presented high specificity in the biopsies of patients with DN (p < 0.0001). Our data suggest that Mindin may play a role in DN pathogenesis and is a promising biomarker of podocyte lesions.
Copyright: © 2023 Martins et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures



References
-
- Narres M, Claessen H, Droste S, Kvitkina T, Koch M, Kuss O, et al.. The Incidence of End-Stage Renal Disease in the Diabetic (Compared to the Non-Diabetic) Population: A Systematic Review. PLoS One. 2016;11(1):e0147329. Epub 20160126. doi: 10.1371/journal.pone.0147329 ; PubMed Central PMCID: PMC4727808. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

