An Sfi1-like centrin-interacting centriolar plaque protein affects nuclear microtubule homeostasis
- PMID: 37130129
- PMCID: PMC10180636
- DOI: 10.1371/journal.ppat.1011325
An Sfi1-like centrin-interacting centriolar plaque protein affects nuclear microtubule homeostasis
Abstract
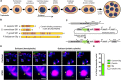
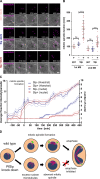
Malaria-causing parasites achieve rapid proliferation in human blood through multiple rounds of asynchronous nuclear division followed by daughter cell formation. Nuclear divisions critically depend on the centriolar plaque, which organizes intranuclear spindle microtubules. The centriolar plaque consists of an extranuclear compartment, which is connected via a nuclear pore-like structure to a chromatin-free intranuclear compartment. Composition and function of this non-canonical centrosome remain largely elusive. Centrins, which reside in the extranuclear part, are among the very few centrosomal proteins conserved in Plasmodium falciparum. Here we identify a novel centrin-interacting centriolar plaque protein. Conditional knock down of this Sfi1-like protein (PfSlp) caused a growth delay in blood stages, which correlated with a reduced number of daughter cells. Surprisingly, intranuclear tubulin abundance was significantly increased, which raises the hypothesis that the centriolar plaque might be implicated in regulating tubulin levels. Disruption of tubulin homeostasis caused excess microtubules and aberrant mitotic spindles. Time-lapse microscopy revealed that this prevented or delayed mitotic spindle extension but did not significantly interfere with DNA replication. Our study thereby identifies a novel extranuclear centriolar plaque factor and establishes a functional link to the intranuclear compartment of this divergent eukaryotic centrosome.
Copyright: © 2023 Wenz et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures


Similar articles
-
Disruption of Plasmodium falciparum kinetochore proteins destabilises the nexus between the centrosome equivalent and the mitotic apparatus.Nat Commun. 2024 Jul 10;15(1):5794. doi: 10.1038/s41467-024-50167-6. Nat Commun. 2024. PMID: 38987258 Free PMC article.
-
An extended DNA-free intranuclear compartment organizes centrosome microtubules in malaria parasites.Life Sci Alliance. 2021 Sep 17;4(11):e202101199. doi: 10.26508/lsa.202101199. Print 2021 Nov. Life Sci Alliance. 2021. PMID: 34535568 Free PMC article.
-
Plasmodium centrin PbCEN-4 localizes to the putative MTOC and is dispensable for malaria parasite proliferation.Biol Open. 2019 Jan 29;8(1):bio036822. doi: 10.1242/bio.036822. Biol Open. 2019. PMID: 30541825 Free PMC article.
-
Big Lessons from Little Yeast: Budding and Fission Yeast Centrosome Structure, Duplication, and Function.Annu Rev Genet. 2017 Nov 27;51:361-383. doi: 10.1146/annurev-genet-120116-024733. Epub 2017 Sep 15. Annu Rev Genet. 2017. PMID: 28934593 Review.
-
Regulation of microtubule nucleation mediated by γ-tubulin complexes.Protoplasma. 2017 May;254(3):1187-1199. doi: 10.1007/s00709-016-1070-z. Epub 2017 Jan 10. Protoplasma. 2017. PMID: 28074286 Review.
Cited by
-
Insights into the Cell Division of Neospora caninum.Microorganisms. 2023 Dec 28;12(1):61. doi: 10.3390/microorganisms12010061. Microorganisms. 2023. PMID: 38257886 Free PMC article.
-
A dynamic barrier: remodeling of the nuclear envelope during closed mitosis in malaria parasites.mSphere. 2025 Jul 29;10(7):e0099924. doi: 10.1128/msphere.00999-24. Epub 2025 Jun 9. mSphere. 2025. PMID: 40488521 Free PMC article. Review.
-
The molecular mechanisms driving Plasmodium cell division.Biochem Soc Trans. 2024 Apr 24;52(2):593-602. doi: 10.1042/BST20230403. Biochem Soc Trans. 2024. PMID: 38563493 Free PMC article. Review.
-
Disruption of Plasmodium falciparum kinetochore proteins destabilises the nexus between the centrosome equivalent and the mitotic apparatus.Nat Commun. 2024 Jul 10;15(1):5794. doi: 10.1038/s41467-024-50167-6. Nat Commun. 2024. PMID: 38987258 Free PMC article.
-
Malaria parasite centrins can assemble by Ca2+-inducible condensation.PLoS Pathog. 2023 Dec 27;19(12):e1011899. doi: 10.1371/journal.ppat.1011899. eCollection 2023 Dec. PLoS Pathog. 2023. PMID: 38150475 Free PMC article.
References
-
- WHO. WHO Global, World Malaria Report 2021. Word Malaria report Geneva: World Health Organization. (2021). Licence: CC. 2021.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

