Validation of a rapid SARS-CoV-2 antibody test in general practice
- PMID: 37130685
- PMCID: PMC10163333
- DOI: 10.1136/bmjopen-2022-069997
Validation of a rapid SARS-CoV-2 antibody test in general practice
Abstract
Objectives: To validate a rapid serological test (RST) for SARS-CoV-2 antibodies used in seroprevalence studies in healthcare providers, including primary healthcare providers (PHCPs) in Belgium.
Design: A phase III validation study of the RST (OrientGene) within a prospective cohort study.
Setting: Primary care in Belgium.
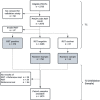
Participants: Any general practitioner (GP) working in primary care in Belgium and any other PHCP from the same GP practice who physically manages patients were eligible in the seroprevalence study. For the validation study, all participants who tested positive (376) on the RST at the first testing timepoint (T1) and a random sample of those who tested negative (790) and unclear (24) were included.
Intervention: At T2, 4 weeks later, PHCPs performed the RST with fingerprick blood (index test) immediately after providing a serum sample to be analysed for the presence of SARS-CoV-2 immunoglobulin G antibodies using a two-out-of-three assay (reference test).
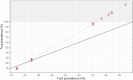
Primary and secondary outcome measures: The RST accuracy was estimated using inverse probability weighting to correct for missing reference test data, and considering unclear RST results as negative for the sensitivity and positive for the specificity. Using these conservative estimates, the true seroprevalence was estimated both for T2 and RST-based prevalence values found in a cohort study with PHCPs in Belgium.
Results: 1073 paired tests (403 positive on the reference test) were included. A sensitivity of 73% (a specificity of 92%) was found considering unclear RST results as negative (positive). For an RST-based prevalence at T1 (13.9), T2 (24.9) and T7 (70.21), the true prevalence was estimated to be 9.1%, 25.9% and 95.7%, respectively.
Conclusion: The RST sensitivity (73%) and specificity (92%) make an RST-based seroprevalence below (above) 23% overestimate (underestimate) the true seroprevalence.
Trial registration number: NCT04779424.
Keywords: COVID-19; infectious diseases; primary care.
© Author(s) (or their employer(s)) 2023. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: None declared.
Figures
References
-
- Roser M, Ritchie H, Ortiz-Ospina E, et al. . Coronavirus pandemic (COVID-19). our world in data. 2020. Available: https://ourworldindata.org/coronavirus
-
- Adriaenssens N, Scholtes B, Bruyndonckx R, et al. . Prevalence and incidence of antibodies against SARS-cov-2 among primary healthcare providers in Belgium during 1 year of the COVID-19 epidemic: prospective cohort study protocol. BMJ Open 2022;12:e054688. 10.1136/bmjopen-2021-054688 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous
