Association of serum neurofilament light with microglial activation in multiple sclerosis
- PMID: 37130728
- PMCID: PMC10447382
- DOI: 10.1136/jnnp-2023-331051
Association of serum neurofilament light with microglial activation in multiple sclerosis
Abstract
Background: Translocator protein (TSPO)-PET and neurofilament light (NfL) both report on brain pathology, but their potential association has not yet been studied in multiple sclerosis (MS) in vivo. We aimed to evaluate the association between serum NfL (sNfL) and TSPO-PET-measurable microglial activation in the brain of patients with MS.
Methods: Microglial activation was detected using PET and the TSPO-binding radioligand [11C]PK11195. Distribution volume ratio (DVR) was used to evaluate specific [11C]PK11195-binding. sNfL levels were measured using single molecule array (Simoa). The associations between [11C]PK11195 DVR and sNfL were evaluated using correlation analyses and false discovery rate (FDR) corrected linear regression modelling.
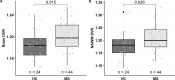
Results: 44 patients with MS (40 relapsing-remitting and 4 secondary progressive) and 24 age-matched and sex-matched healthy controls were included. In the patient group with elevated brain [11C]PK11195 DVR (n=19), increased sNfL associated with higher DVR in the lesion rim (estimate (95% CI) 0.49 (0.15 to 0.83), p(FDR)=0.04) and perilesional normal appearing white matter (0.48 (0.14 to 0.83), p(FDR)=0.04), and with a higher number and larger volume of TSPO-PET-detectable rim-active lesions defined by microglial activation at the plaque edge (0.46 (0.10 to 0.81), p(FDR)=0.04 and 0.50 (0.17 to 0.84), p(FDR)=0.04, respectively). Based on the multivariate stepwise linear regression model, the volume of rim-active lesions was the most relevant factor affecting sNfL.
Conclusions: Our demonstration of an association between microglial activation as measured by increased TSPO-PET signal, and elevated sNfL emphasises the significance of smouldering inflammation for progression-promoting pathology in MS and highlights the role of rim-active lesions in promoting neuroaxonal damage.
Keywords: Microglia; Multiple Sclerosis; Neurofilament light; PET; TSPO.
© Author(s) (or their employer(s)) 2023. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: MSa has received support for attending meetings and/or travel from Turku University Foundation, the InFLAMES Flagship Programme of the Academy of Finland and Merck. MM has no competing interests. AV has received a personal grants from Päivikki and Sakari Sohlberg Foundation and Janssen Pharmaceutica. SL has received research support from the Turunmaa Duodecim Society, Finnish Brain Foundation and Turku Doctoral Programme in Clinical Research, and travel honoraria from Turku University Foundation and Turku Doctoral Programme in Clinical Research. MSu has received research support from The Finnish Medical Foundation, The Finnish MS Foundation and from The Finnish Medical Society. DL is chief medical officer of GeNeuro. JK has received speaker fees, research support, travel support and/or served on advisory boards by the Progressive MS Alliance, Swiss MS Society, Swiss National Research Foundation (320030_189140 / 1), University of Basel, Biogen, Celgene, Merck, Novartis, Octave Bioscience, Roche, Sanofi. LA has received institutional research support (grants) from the Academy of Finland, Sigrid Juselius Foundation, Sanofi-Genzyme, Merck and Novartis and honoraria for lectures and/or for advising from Novartis, Sanofi Genzyme, Janssen, Merck and ParadigMS Foundation, and has participated on Novartis scientific advisory board.
Figures



References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
