Somatic Integration of Incoherent Dendritic Inputs in the Gerbil Medial Superior Olive
- PMID: 37130779
- PMCID: PMC10255013
- DOI: 10.1523/JNEUROSCI.2215-22.2023
Somatic Integration of Incoherent Dendritic Inputs in the Gerbil Medial Superior Olive
Abstract
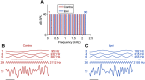
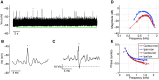
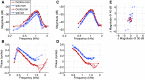
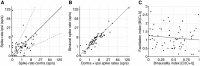
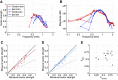
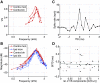
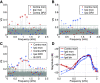
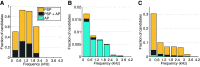
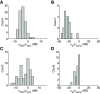
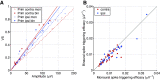
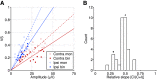
The medial superior olive (MSO) is a binaural nucleus that is specialized in detecting the relative arrival times of sounds at both ears. Excitatory inputs to its neurons originating from either ear are segregated to different dendrites. To study the integration of synaptic inputs both within and between dendrites, we made juxtacellular and whole-cell recordings from the MSO in anesthetized female gerbils, while presenting a "double zwuis" stimulus, in which each ear received its own set of tones, which were chosen in a way that all second-order distortion products (DP2s) could be uniquely identified. MSO neurons phase-locked to multiple tones within the multitone stimulus, and vector strength, a measure for spike phase-locking, generally depended linearly on the size of the average subthreshold response to a tone. Subthreshold responses to tones in one ear depended little on the presence of sound in the other ear, suggesting that inputs from different ears sum linearly without a substantial role for somatic inhibition. The "double zwuis" stimulus also evoked response components in the MSO neuron that were phase-locked to DP2s. Bidendritic subthreshold DP2s were quite rare compared with bidendritic suprathreshold DP2s. We observed that in a small subset of cells, the ability to trigger spikes differed substantially between both ears, which might be explained by a dendritic axonal origin. Some neurons that were driven monaurally by only one of the two ears nevertheless showed decent binaural tuning. We conclude that MSO neurons are remarkably good in finding binaural coincidences even among uncorrelated inputs.SIGNIFICANCE STATEMENT Neurons in the medial superior olive are essential for precisely localizing low-frequency sounds in the horizontal plane. From their soma, only two dendrites emerge, which are innervated by inputs originating from different ears. Using a new sound stimulus, we studied the integration of inputs both within and between these dendrites in unprecedented detail. We found evidence that inputs from different dendrites add linearly at the soma, but that small increases in somatic potentials could lead to large increases in the probability of generating a spike. This basic scheme allowed the MSO neurons to detect the relative arrival time of inputs at both dendrites remarkably efficient, although the relative size of these inputs could differ considerably.
Keywords: binaural coincidences; distortion products; excitatory inputs; phase-locking; sound localization; vector strength.
Copyright © 2023 the authors.
Figures



References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
