Development of Bifunctional, Raman Active Diyne-Girder Stapled α-Helical Peptides
- PMID: 37130830
- PMCID: PMC10946806
- DOI: 10.1002/chem.202300855
Development of Bifunctional, Raman Active Diyne-Girder Stapled α-Helical Peptides
Abstract
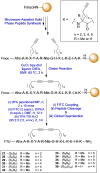
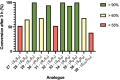
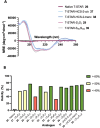
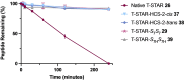
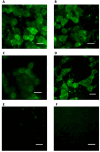
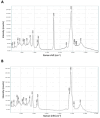
Stapled peptides are a unique class of cyclic α-helical peptides that are conformationally constrained via their amino acid side-chains. They have been transformative to the field of chemical biology and peptide drug discovery through addressing many of the physicochemical limitations of linear peptides. However, there are several issues with current chemical strategies to produce stapled peptides. For example, two distinct unnatural amino acids are required to synthesize i, i+7 alkene stapled peptides, leading to high production costs. Furthermore, low purified yields are obtained due to cis/trans isomers produced during ring-closing metathesis macrocyclisation. Here we report the development of a new i, i+7 diyne-girder stapling strategy that addresses these issues. The asymmetric synthesis of nine unnatural Fmoc-protected alkyne-amino acids facilitated a systematic study to determine the optimal (S,S)-stereochemistry and 14-carbon diyne-girder bridge length. Diyne-girder stapled T-STAR peptide 29 was demonstrated to have excellent helicity, cell permeability and stability to protease degradation. Finally, we demonstrate that the diyne-girder constraint is a Raman chromophore with potential use in Raman cell microscopy. Development of this highly effective, bifunctional diyne-girder stapling strategy leads us to believe that it can be used to produce other stapled peptide probes and therapeutics.
Keywords: Raman active; bifunctional; protease stable; stapled peptide; α-helical.
© 2023 The Authors. Chemistry - A European Journal published by Wiley-VCH GmbH.
Conflict of interest statement
A patent application [(GB) Patent Application No: 2219576.2] has been submitted on this work.
Figures


References
MeSH terms
Substances
Grants and funding
- EP/L018152/1/Engineering and Physical Sciences Research Council
- EP/N509668/1/Engineering and Physical Sciences Research Council
- EP/R513222/1/Engineering and Physical Sciences Research Council
- DSTL/AGR/ R/CBRN/01/Defence Science and Technology Laboratory
- DSTLX-1000141308/Defence Science and Technology Laboratory

