Melatonin and erastin emerge synergistic anti-tumor effects on oral squamous cell carcinoma by inducing apoptosis, ferroptosis, and inhibiting autophagy through promoting ROS
- PMID: 37131152
- PMCID: PMC10155313
- DOI: 10.1186/s11658-023-00449-6
Melatonin and erastin emerge synergistic anti-tumor effects on oral squamous cell carcinoma by inducing apoptosis, ferroptosis, and inhibiting autophagy through promoting ROS
Abstract
Background: Oral squamous cell carcinomas are one of the most common cancers worldwide with aggressive behavior and poor prognosis. Reactive oxygen species (ROS) are associated with cancer and cause various types of regulated cell death (RCD). Inducing the RCD pathway by modulating ROS levels is imperative to conquer cancers. The aim of this study is to investigate the synergistic anticancer effects of melatonin and erastin on ROS modulation and subsequent RCD induction.
Methods: Human tongue squamous cell carcinoma cell lines (SCC-15 cells) were treated with melatonin, erastin, or their combination. Cell viability, ROS levels, autophagy, apoptosis, and ferroptosis levels were tested according to the results of the PCR array, which were verified with/without the induction and inhibition of ROS by H2O2 and N-acetyl-L-cysteine, respectively. In addition, a mouse-based subcutaneous oral cancer xenograft model was constructed to identify the effects of melatonin, erastin, and their combination on the autophagy, apoptosis, and ferroptosis levels in isolated tumor tissues.
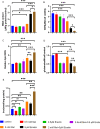
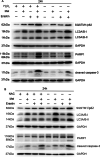
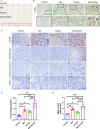
Results: ROS levels were increased by the administration of melatonin at high concentrations (mM), and the combination of melatonin with erastin enhanced the levels of malonic dialdehyde, ROS, and lipid ROS, and reduced the levels of glutamate and glutathione. SQSTM1/p62, LC3A/B, cleaved caspase-3, and PARP1 protein levels in SCC-15 cells were also increased by melatonin plus erastin treatment, which further increased as ROS accumulated, and decreased as ROS levels were suppressed. Combined treatment of melatonin and erastin markedly reduced the tumor size in vivo, demonstrated no obvious systemic side effects, and significantly enhanced the apoptosis and ferroptosis levels in the tumor tissues, in parallel with decreased autophagy levels.
Conclusions: Melatonin combined with erastin exhibits synergistic anticancer effects without adverse reactions. Herein, this combination might become a promising alternative strategy for oral cancer treatment.
Keywords: Apoptosis; Autophagy; Erastin; Ferroptosis; Melatonin; Oral cancer; Reactive oxygen species (ROS).
© 2023. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures


References
-
- Jansen L, Buttmann-Schweiger N, Listl S, Ressing M, Holleczek B, Katalinic A, et al. Differences in incidence and survival of oral cavity and pharyngeal cancers between Germany and the United States depend on the HPV-association of the cancer site. Oral Oncol. 2018;76:8–15. doi: 10.1016/j.oraloncology.2017.11.015. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

