This is a preprint.
A novel injectable radiopaque hydrogel with potent properties for multicolor CT imaging in the context of brain and cartilage regenerative therapy
- PMID: 37131613
- PMCID: PMC10153246
- DOI: 10.1101/2023.04.20.537520
A novel injectable radiopaque hydrogel with potent properties for multicolor CT imaging in the context of brain and cartilage regenerative therapy
Abstract
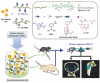
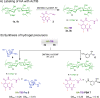
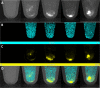
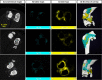
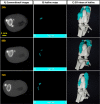
Cell therapy is promising to treat many conditions, including neurological and osteoarticular diseases. Encapsulation of cells within hydrogels facilitates cell delivery and can improve therapeutic effects. However, much work remains to be done to align treatment strategies with specific diseases. The development of imaging tools that enable monitoring cells and hydrogel independently is key to achieving this goal. Our objective herein is to longitudinally study an iodine-labeled hydrogel, incorporating gold-labeled stem cells, by bicolor CT imaging after in vivo injection in rodent brains or knees. To this aim, an injectable self-healing hyaluronic acid (HA) hydrogel with long-persistent radiopacity was formed by the covalent grafting of a clinical contrast agent on HA. The labeling conditions were tuned to achieve sufficient X-ray signal and to maintain the mechanical and self-healing properties as well as injectability of the original HA scaffold. The efficient delivery of both cells and hydrogel at the targeted sites was demonstrated by synchrotron K-edge subtraction-CT. The iodine labeling enabled to monitor the hydrogel biodistribution in vivo up to 3 days post-administration, which represents a technological first in the field of molecular CT imaging agents. This tool may foster the translation of combined cell-hydrogel therapies into the clinics.
Keywords: Hyaluronic acid; bicolor X-ray imaging techniques; cell therapy; injectable hydrogel; iodine contrast agent.
Figures





References
-
- Boisserand Ligia S. B., Kodama T., Papassin J., Auzely R., Moisan A., Rome C., Detante O., Stem cells international 2016, 2016, 6810562, 10.1155/2016/6810562; - DOI - PMC - PubMed
- Liu Y., Wang M., Luo Y., Liang Q., Yu Y., Chen F., Yao J., Gels 2021, 7 (4), 263, 10.3390/gels7040263; - DOI - PMC - PubMed
- Marquardt L. M., Heilshorn S. C., Curr Stem Cell Rep 2016, 2 (3), 207, 10.1007/s40778-016-0058-0; - DOI - PMC - PubMed
- d) Totten J. D., Alhadrami H. A., Jiffri E. H., McMullen C. J., Seib F. P., Carswell H. V. O., Trends in Biotechnology 2022, 40 (6), 708, 10.1016/j.tibtech.2021.10.009. - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources
