This is a preprint.
Outpatient COVID-19 convalescent plasma recipient antibody thresholds correlated to reduced hospitalizations within a randomized trial
- PMID: 37131659
- PMCID: PMC10153328
- DOI: 10.1101/2023.04.13.23288353
Outpatient COVID-19 convalescent plasma recipient antibody thresholds correlated to reduced hospitalizations within a randomized trial
Update in
-
Outpatient COVID-19 convalescent plasma recipient antibody thresholds correlated to reduced hospitalizations within a randomized trial.JCI Insight. 2024 Mar 14;9(8):e178460. doi: 10.1172/jci.insight.178460. JCI Insight. 2024. PMID: 38483534 Free PMC article. Clinical Trial.
Abstract
Background: The COVID-19 convalescent plasma (CCP) viral specific antibody levels that translate into recipient post-transfusion antibody levels sufficient to prevent disease progression is not defined.
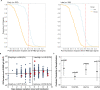
Methods: This secondary analysis correlated donor and recipient antibody levels to hospitalization risk among unvaccinated, seronegative CCP recipients within the outpatient, double blind, randomized clinical trial that compared CCP to control plasma. The majority of COVID-19 CCP arm hospitalizations (15/17, 88%) occurred in this unvaccinated, seronegative subgroup. A functional cutoff to delineate recipient high versus low post-transfusion antibody levels was established by two methods: 1) analyzing virus neutralization-equivalent anti-S-RBD IgG responses in donors or 2) receiver operating characteristic (ROC) analysis.
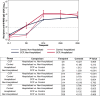
Results: SARS-CoV-2 anti-S-RBD IgG antibody was diluted by a factor of 21.3 into post-transfusion seronegative recipients from matched donor units. Viral specific antibody delivered approximated 1.2 mg. The high antibody recipients transfused early (symptom onset within 5 days) had no hospitalizations. A CCP recipient analysis for antibody thresholds correlated to reduced hospitalizations found a significant association with Fisher's exact test between early and high antibodies versus all other CCP recipients (or control plasma) with antibody cutoffs established by both methods-donor virus neutralization-based cutoff: (0/85; 0% versus 15/276; 5.6%) p=0.03 or ROC based cutoff: (0/94; 0% versus 15/267; 5.4%) p=0.01.
Conclusion: In unvaccinated, seronegative CCP recipients, early transfusion of plasma units corresponding to the upper 30% of all study donors reduced outpatient hospitalizations. These high antibody level plasma units, given early, should be reserved for therapeutic use.Trial registration: NCT04373460.
Funding: Defense Health Agency and others.
Keywords: COVID-19; convalescent plasma; hospitalization; outpatients; randomized controlled trial; viral load.
Conflict of interest statement
Conflict of Interest Statement TG-Paid consultant and employee of Fenwal, a Fresenius Kabi company; AC-Scientific Advisory Board of Sabtherapeutics (cow-derived human immunoglobulins COVID-19 treatment and other infectious diseases) and Ortho Diagnostics Speakers Bureau; MAH-contracts from Gilead Sciences, Insmed, AN2 Therapeutics, AstraZeneca to the University of Cincinnati, outside the submitted work. EB-member of the FDA Blood Products Advisory Committee; SS reports research grants; F2G, Cidara, Ansun, Zeteo: personal fees as consultant, advisory board, data safety monitoring board member; Celltrion, Adagio, Immunome, Karius, Pfizer, Scynexis, Adamis, Karyopharm, Intermountain Health: Stock options: Immunome; CS: Centers for Disease Control and Prevention, Merck, Pfizer: Research Grants. All other authors report no relevant disclosures.
Figures


References
-
- Zhang S, Ma P, Orzechowski M, Lemmer A, Rzasa K, Bagnall J, et al. High-Throughput Neutralization and Serology Assays Reveal Correlated but Highly Variable Humoral Immune Responses in a Large Population of Individuals Infected with SARS-CoV-2 in the US between March and August 2020. mBio. 2023;14(2):e0352322. - PMC - PubMed
-
- Graninger M, Jani CM, Reuberger E, Pruger K, Gaspar P, Springer DN, et al. Comprehensive Comparison of Seven SARS-CoV-2-Specific Surrogate Virus Neutralization and Anti-Spike IgG Antibody Assays Using a Live-Virus Neutralization Assay as a Reference. Microbiol Spectr. 2023;11(1):e0231422. - PMC - PubMed
Publication types
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous
