This is a preprint.
Structure and assembly of a bacterial gasdermin pore
- PMID: 37131678
- PMCID: PMC10153256
- DOI: 10.1101/2023.04.20.537723
Structure and assembly of a bacterial gasdermin pore
Update in
-
Structure and assembly of a bacterial gasdermin pore.Nature. 2024 Apr;628(8008):657-663. doi: 10.1038/s41586-024-07216-3. Epub 2024 Mar 20. Nature. 2024. PMID: 38509367 Free PMC article.
Abstract
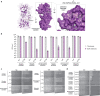
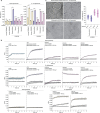
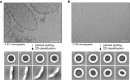
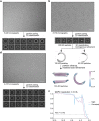
In response to pathogen infection, gasdermin (GSDM) proteins form membrane pores that induce a host cell death process called pyroptosis1-33. Studies of human and mouse GSDM pores reveal the functions and architectures of 24-33 protomers assemblies4-9, but the mechanism and evolutionary origin of membrane targeting and GSDM pore formation remain unknown. Here we determine a structure of a bacterial GSDM (bGSDM) pore and define a conserved mechanism of pore assembly. Engineering a panel of bGSDMs for site-specific proteolytic activation, we demonstrate that diverse bGSDMs form distinct pore sizes that range from smaller mammalian-like assemblies to exceptionally large pores containing >50 protomers. We determine a 3.3 Å cryo-EM structure of a Vitiosangium bGSDM in an active slinky-like oligomeric conformation and analyze bGSDM pores in a native lipid environment to create an atomic-level model of a full 52-mer bGSDM pore. Combining our structural analysis with molecular dynamics simulations and cellular assays, our results support a stepwise model of GSDM pore assembly and suggest that a covalently bound palmitoyl can leave a hydrophobic sheath and insert into the membrane before formation of the membrane-spanning β-strand regions. These results reveal the diversity of GSDM pores found in nature and explain the function of an ancient post-translational modification in enabling programmed host cell death.
Conflict of interest statement
Competing Interests None declared.
Figures













References
-
- Broz P., Pelegrín P. & Shao F. The gasdermins, a protein family executing cell death and inflammation. Nat. Rev. Immunol. 20, 143–157 (2020). - PubMed
-
- Shi J., Gao W. & Shao F. Pyroptosis: Gasdermin-Mediated Programmed Necrotic Cell Death. Trends Biochem. Sci. 42, 245–254 (2017). - PubMed
-
- Ding J. et al. Pore-forming activity and structural autoinhibition of the gasdermin family. Nature 535, 111–116 (2016). - PubMed
Extended References
-
- Punjani A., Rubinstein J. L., Fleet D. J. & Brubaker M. A. CryoSPARC: Algorithms for rapid unsupervised cryo-EM structure determination. Nat. Methods 14, 290–296 (2017). - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
