This is a preprint.
"Metformin Impairs Intestinal Fructose Metabolism"
- PMID: 37131695
- PMCID: PMC10153158
- DOI: 10.1101/2023.04.17.537251
"Metformin Impairs Intestinal Fructose Metabolism"
Abstract
Objective: To investigate the effects of metformin on intestinal carbohydrate metabolism in vivo.
Method: Male mice preconditioned with a high-fat, high-sucrose diet were treated orally with metformin or a control solution for two weeks. Fructose metabolism, glucose production from fructose, and production of other fructose-derived metabolites were assessed using stably labeled fructose as a tracer.
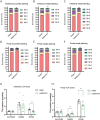
Results: Metformin treatment decreased intestinal glucose levels and reduced incorporation of fructose-derived metabolites into glucose. This was associated with decreased intestinal fructose metabolism as indicated by decreased enterocyte F1P levels and diminished labeling of fructose-derived metabolites. Metformin also reduced fructose delivery to the liver. Proteomic analysis revealed that metformin coordinately down-regulated proteins involved carbohydrate metabolism including those involved in fructolysis and glucose production within intestinal tissue.
Conclusion: Metformin reduces intestinal fructose metabolism, and this is associated with broad-based changes in intestinal enzyme and protein levels involved in sugar metabolism indicating that metformin's effects on sugar metabolism are pleiotropic.
Keywords: Metformin; fructose metabolism; glucose production; isotope-tracing metabolomics; proteomics.
Figures





References
-
- Sanchez-Rangel E., Inzucchi S.E., 2017. Metformin: clinical use in type 2 diabetes. Diabetologia 60(9):1586–1593. - PubMed
-
- Lin H.Z., Yang S.Q., Chuckaree C., Kuhajda F., Ronnet G., Diehl A.M., 2000. Metformin reverses fatty liver disease in obese, leptin-deficient mice. Nat Med 6(9):998–1003. - PubMed
-
- Farr S.A., Roesler E., Niehoff M.L., Roby D.A., McKee A., Morley J.E., 2019. Metformin Improves Learning and Memory in the SAMP8 Mouse Model of Alzheimer's Disease. J Alzheimers Dis 68(4):1699–1710. - PubMed
-
- Cerezo M., Tichet M., Abbe P., Ohanna M., Lehraiki A., Rouaud F., et al., 2013. Metformin blocks melanoma invasion and metastasis development in AMPK/p53-dependent manner. Mol Cancer Ther 12(8):1605–1615. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
