This is a preprint.
Metabolism of host lysophosphatidylcholine in Plasmodium falciparum-infected erythrocytes
- PMID: 37131712
- PMCID: PMC10153170
- DOI: 10.1101/2023.04.17.537066
Metabolism of host lysophosphatidylcholine in Plasmodium falciparum-infected erythrocytes
Update in
-
Metabolism of host lysophosphatidylcholine in Plasmodium falciparum-infected erythrocytes.Proc Natl Acad Sci U S A. 2024 Feb 20;121(8):e2320262121. doi: 10.1073/pnas.2320262121. Epub 2024 Feb 13. Proc Natl Acad Sci U S A. 2024. PMID: 38349879 Free PMC article.
Abstract
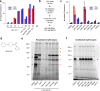
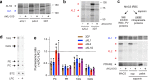
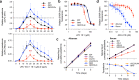
The human malaria parasite Plasmodium falciparum requires exogenous fatty acids to support its growth during the pathogenic, asexual erythrocytic stage. Host serum lysophosphatidylcholine (LPC) is a significant fatty acid source, yet the metabolic processes responsible for the liberation of free fatty acids from exogenous LPC are unknown. Using a novel assay for LPC hydrolysis in P. falciparum-infected erythrocytes, we have identified small-molecule inhibitors of key in situ lysophospholipase activities. Competitive activity-based profiling and generation of a panel of single-to-quadruple knockout parasite lines revealed that two enzymes of the serine hydrolase superfamily, termed exported lipase (XL) 2 and exported lipase homolog (XLH) 4, are the dominant lysophospholipase activities in parasite-infected erythrocytes. The parasite ensures efficient exogenous LPC hydrolysis by directing these two enzymes to distinct locations: XL2 is exported to the erythrocyte, while XLH4 is retained within the parasite. While XL2 and XLH4 were individually dispensable with little effect on LPC hydrolysis in situ, loss of both enzymes resulted in a strong reduction in fatty acid scavenging from LPC, hyperproduction of phosphatidylcholine, and an enhanced sensitivity to LPC toxicity. Notably, growth of XL/XLH-deficient parasites was severely impaired when cultured in media containing LPC as the sole exogenous fatty acid source. Furthermore, when XL2 and XLH4 activities were ablated by genetic or pharmacologic means, parasites were unable to proliferate in human serum, a physiologically-relevant fatty acid source, revealing the essentiality of LPC hydrolysis in the host environment and its potential as a target for anti-malarial therapy.
Figures

References
-
- WHO World malaria report 2022. (2022).
-
- Vial H.J. & Ancelin M.L. Malarial lipids, in Malaria: parasite biology, biogenesis, protection. (ed. Sherman I.W.) (American Association of Microbiology Press, Washington, DC; 1998).
-
- Palacpac N.M.Q. et al. Developmental-stage-specific triacylglycerol biosynthesis, degradation and trafficking as lipid bodies in Plasmodium falciparum-infected erythrocytes. J. Cell Sci. 117, 1469–1480 (2004). - PubMed
-
- Jackson K.E. et al. Food vacuole-associated lipid bodies and heterogeneous lipid environments in the malaria parasite, Plasmodium falciparum. Mol. Microbiol. 54, 109–122 (2004). - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
