CINeMA: Software for semiautomated assessment of the confidence in the results of network meta-analysis
- PMID: 37131978
- PMCID: PMC8356302
- DOI: 10.1002/cl2.1080
CINeMA: Software for semiautomated assessment of the confidence in the results of network meta-analysis
Abstract
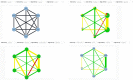
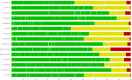
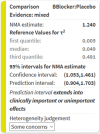
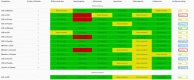
Network meta-analysis (NMA) compares several interventions that are linked in a network of comparative studies and estimates the relative treatment effects between all treatments, using both direct and indirect evidence. NMA is increasingly used for decision making in health care, however, a user-friendly system to evaluate the confidence that can be placed in the results of NMA is currently lacking. This paper is a tutorial describing the Confidence In Network Meta-Analysis (CINeMA) web application, which is based on the framework developed by Salanti et al (2014, PLOS One, 9, e99682) and refined by Nikolakopoulou et al (2019, bioRxiv). Six domains that affect the level of confidence in the NMA results are considered: (a) within-study bias, (b) reporting bias, (c) indirectness, (d) imprecision, (e) heterogeneity, and (f) incoherence. CINeMA is freely available and open-source and no login is required. In the configuration step users upload their data, produce network plots and define the analysis and effect measure. The dataset should include assessments of study-level risk of bias and judgments on indirectness. CINeMA calls the netmeta routine in R to estimate relative effects and heterogeneity. Users are then guided through a systematic evaluation of the six domains. In this way reviewers assess the level of concerns for each relative treatment effect from NMA as giving rise to "no concerns," "some concerns," or "major concerns" in each of the six domains, which are graphically summarized on the report page for all effect estimates. Finally, judgments across the domains are summarized into a single confidence rating ("high," "moderate," "low," or "very low"). In conclusion, the user-friendly web-based CINeMA platform provides a transparent framework to evaluate evidence from systematic reviews with multiple interventions.
© 2020 The Authors. Campbell Systematic Reviews published by John Wiley & Sons Ltd on behalf of The Campbell Collaboration.
Figures



References
-
- Chaimani, A. , & Salanti, G. (2012). Using network meta‐analysis to evaluate the existence of small‐study effects in a network of interventions. Research Synthesis Methods, 3(2), 161–176. - PubMed
-
- CINeMA: Confidence in Network Meta‐Analysis [Software] (2017). Institute of Social and Preventive Medicine, University of Bern, Retrieved from: https://cinema.ispm.unibe.ch/
-
- Cipriani, A. , Furukawa, T. A. , Salanti, G. , Chaimani, A. , Atkinson, L. Z. , Ogawa, Y. , … Geddes, J. R. (2018). Comparative efficacy and acceptability of 21 antidepressant drugs for the acute treatment of adults with major depressive disorder: A systematic review and network meta‐analysis. Lancet, 391(10128), 1357–1366. - PMC - PubMed
-
- Dias, S. , Welton, N. J. , Caldwell, D. M. , & Ades, A. E. (2010). Checking consistency in mixed treatment comparison meta‐analysis. Statistics in Medicine, 29(7–8), 932–944. - PubMed
LinkOut - more resources
Full Text Sources
