Molecular mechanisms underlying adverse effects of dexamethasone and betamethasone in the developing cardiovascular system
- PMID: 37132324
- PMCID: PMC10946807
- DOI: 10.1096/fj.202200676RR
Molecular mechanisms underlying adverse effects of dexamethasone and betamethasone in the developing cardiovascular system
Abstract
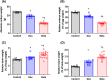
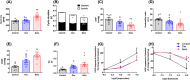
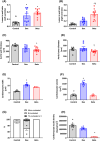
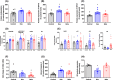
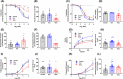
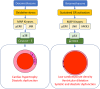
Antenatal glucocorticoids accelerate fetal lung maturation and reduce mortality in preterm babies but can trigger adverse effects on the cardiovascular system. The mechanisms underlying off-target effects of the synthetic glucocorticoids mostly used, Dexamethasone (Dex) and Betamethasone (Beta), are unknown. We investigated effects of Dex and Beta on cardiovascular structure and function, and underlying molecular mechanism using the chicken embryo, an established model system to isolate effects of therapy on the developing heart and vasculature, independent of effects on the mother or placenta. Fertilized eggs were treated with Dex (0.1 mg kg-1 ), Beta (0.1 mg kg-1 ), or water vehicle (Control) on embryonic day 14 (E14, term = 21 days). At E19, biometry, cardiovascular function, stereological, and molecular analyses were determined. Both glucocorticoids promoted growth restriction, with Beta being more severe. Beta compared with Dex induced greater cardiac diastolic dysfunction and also impaired systolic function. While Dex triggered cardiomyocyte hypertrophy, Beta promoted a decrease in cardiomyocyte number. Molecular changes of Dex on the developing heart included oxidative stress, activation of p38, and cleaved caspase 3. In contrast, impaired GR downregulation, activation of p53, p16, and MKK3 coupled with CDK2 transcriptional repression linked the effects of Beta on cardiomyocyte senescence. Beta but not Dex impaired NO-dependent relaxation of peripheral resistance arteries. Beta diminished contractile responses to potassium and phenylephrine, but Dex enhanced peripheral constrictor reactivity to endothelin-1. We conclude that Dex and Beta have direct differential detrimental effects on the developing cardiovascular system.
© 2023 The Authors. The FASEB Journal published by Wiley Periodicals LLC on behalf of Federation of American Societies for Experimental Biology.
Figures

Similar articles
-
Isolating adverse effects of glucocorticoids on the embryonic cardiovascular system.FASEB J. 2020 Jul;34(7):9664-9677. doi: 10.1096/fj.202000697R. Epub 2020 Jun 5. FASEB J. 2020. PMID: 32502311 Free PMC article.
-
Low-dose antenatal betamethasone treatment achieves preterm lung maturation equivalent to that of the World Health Organization dexamethasone regimen but with reduced endocrine disruption in a sheep model of pregnancy.Am J Obstet Gynecol. 2022 Dec;227(6):903.e1-903.e16. doi: 10.1016/j.ajog.2022.06.058. Epub 2022 Jul 2. Am J Obstet Gynecol. 2022. PMID: 35792176
-
A placebo-controlled comparison of effects of repetitive doses of betamethasone and dexamethasone on lung maturation and lung, liver, and body weights of mouse pups.Pediatr Res. 2003 Jan;53(1):98-103. doi: 10.1203/00006450-200301000-00017. Pediatr Res. 2003. PMID: 12508087
-
Dexamethasone use during pregnancy: potential adverse effects on embryonic skeletogenesis.Curr Pharm Des. 2014;20(34):5430-7. doi: 10.2174/1381612820666140205144534. Curr Pharm Des. 2014. PMID: 24502599 Review.
-
Antenatal steroids and the developing brain.Arch Dis Child Fetal Neonatal Ed. 2000 Sep;83(2):F154-7. doi: 10.1136/fn.83.2.f154. Arch Dis Child Fetal Neonatal Ed. 2000. PMID: 10952714 Free PMC article. Review.
Cited by
-
Electrical remodeling of atrioventricular junction: a study on retrogradely perfused chick embryonic heart.Am J Physiol Heart Circ Physiol. 2024 Sep 1;327(3):H555-H564. doi: 10.1152/ajpheart.00115.2024. Epub 2024 Jul 19. Am J Physiol Heart Circ Physiol. 2024. PMID: 39028286 Free PMC article.
-
Antenatal Corticosteroids in Early and Late Fetal Growth Restriction.J Clin Med. 2025 Jul 9;14(14):4876. doi: 10.3390/jcm14144876. J Clin Med. 2025. PMID: 40725567 Free PMC article. Review.
References
-
- NIH . The effect of antenatal steroids for fetal maturation on perinatal outcomes‐interim draft statement. NIH Consens Stat Online. 1994;12:1‐24. - PubMed
-
- Jellyman J, Fletcher A, Fowden A, Giussani D. Glucocorticoid maturation of fetal cardiovascular function. Trends Mol Med. 2020;26:170‐184. - PubMed
-
- Garrud TAC, Giussani DA. Combined antioxidant and glucocorticoid therapy for safer treatment of preterm birth. Trends Endocrinol Metab. 2019;30:258‐269. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

