Orlistat mouth rinse: Using the tongue to deliver antiobesity medication in a double-blind randomized crossover pilot trial
- PMID: 37132340
- PMCID: PMC10330229
- DOI: 10.1111/dom.15101
Orlistat mouth rinse: Using the tongue to deliver antiobesity medication in a double-blind randomized crossover pilot trial
Abstract
Aim: To investigate the effects of an orlistat mouth rinse on the intake of a high-fat meal.
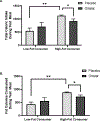
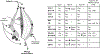
Methods: A double-blind, balanced order, crossover study was conducted in participants (n = 10, body mass index 25-30 kg/m2 ) assigned to receive placebo or orlistat (24 mg/mL) prior to a high-fat meal. Participants were divided into low- or high-fat consumers based on calories consumed from fat following placebo administration.
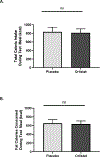
Results: The orlistat mouth rinse decreased total and fat calories consumed during the high-fat meal in high-fat consumers, and did not alter calories consumed in low-fat consumers (P < 0.05).
Conclusions: Orlistat decreases long-chain fatty acid (LCFA) absorption by inhibiting lipases that breakdown triglycerides. Orlistat mouth rinse decreased fat intake in high-fat consumers, suggesting that orlistat inhibited the detection of LCFAs from the high-fat test meal. Lingual delivery of orlistat is predicted to eliminate the risk of oil incontinence and promote weight loss in individuals who prefer fat.
Keywords: fat sensing; obesity; orlistat; tongue.
© 2023 John Wiley & Sons Ltd.
Conflict of interest statement
Figures
Similar articles
-
A rapid and systematic review of the clinical effectiveness and cost-effectiveness of orlistat in the management of obesity.Health Technol Assess. 2001;5(18):1-81. doi: 10.3310/hta5180. Health Technol Assess. 2001. PMID: 11399238
-
Drug interventions for the treatment of obesity in children and adolescents.Cochrane Database Syst Rev. 2016 Nov 29;11(11):CD012436. doi: 10.1002/14651858.CD012436. Cochrane Database Syst Rev. 2016. PMID: 27899001 Free PMC article.
-
What is the clinical effectiveness and cost-effectiveness of using drugs in treating obese patients in primary care? A systematic review.Health Technol Assess. 2012;16(5):iii-xiv, 1-195. doi: 10.3310/hta16050. Health Technol Assess. 2012. PMID: 22340890 Free PMC article.
-
Long-term pharmacotherapy for obesity and overweight.Cochrane Database Syst Rev. 2004;2003(3):CD004094. doi: 10.1002/14651858.CD004094.pub2. Cochrane Database Syst Rev. 2004. PMID: 15266516 Free PMC article.
-
Long-term pharmacotherapy for obesity and overweight.Cochrane Database Syst Rev. 2003;(4):CD004094. doi: 10.1002/14651858.CD004094. Cochrane Database Syst Rev. 2003. Update in: Cochrane Database Syst Rev. 2004;(3):CD004094. doi: 10.1002/14651858.CD004094.pub2. PMID: 14584004 Updated.
Cited by
-
Protective and Therapeutic Effects of Orlistat in Combination with Elettaria cardamomum "Cardamom" Extract on Learning, Memory, Anxiety, and Neuroinflammation in Obese Mice.Medicina (Kaunas). 2025 Feb 4;61(2):263. doi: 10.3390/medicina61020263. Medicina (Kaunas). 2025. PMID: 40005380 Free PMC article.
References
-
- Gila Therapeutics Inc. Gila Therapeutics. [Website]. 2022; https://www.gilatherapeutics.com/. Accessed February 17, 2022.
-
- O’Meara S, Riemsma R, Shirran L, Mather L, ter Riet G. A systematic review of the clinical effectiveness of orlistat used for the management of obesity. Obes Rev. 2004;5(1):51–68. - PubMed
-
- Bansal AM, Khalili YA. LiverTox: Clinical and Research Information on Drug-Induced Liver Injury. Orlistat. Orlistat [Internet]. 2020; https://www.ncbi.nlm.nih.gov/books/NBK548898/?report=reader#!po=91.6667. Accessed February 17, 2022.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

