Membrane-Bound Vimentin Filaments Reorganize and Elongate under Strain
- PMID: 37132386
- PMCID: PMC10265653
- DOI: 10.1021/acs.biomac.3c00025
Membrane-Bound Vimentin Filaments Reorganize and Elongate under Strain
Abstract
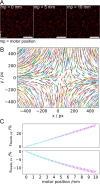
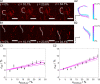
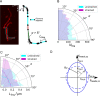
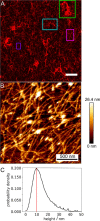
Within a cell, intermediate filaments interact with other cytoskeletal components, altogether providing the cell's mechanical stability. However, little attention has been drawn to intermediate filaments close to the plasma membrane. In this cortex configuration, the filaments are coupled and arranged in parallel to the membrane, and the question arises of how they react to the mechanical stretching of the membrane. To address this question, we set out to establish an in vitro system composed of a polydimethylsiloxane-supported lipid bilayer. With a uniaxial stretching device, the supported membrane was stretched up to 34% in the presence of a lipid reservoir that was provided by adding small unilamellar vesicles in the solution. After vimentin attachment to the membrane, we observed structural changes of the vimentin filaments in networks of different densities by fluorescence microscopy and atomic force microscopy. We found that individual filaments respond to the membrane stretching with a reorganization along the stretching direction as well as an intrinsic elongation, while in a dense network, mainly filament reorganization was observed.
Conflict of interest statement
The authors declare no competing financial interest.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

