Novel Insights on Ferroptosis Modulation as Potential Strategy for Cancer Treatment: When Nature Kills
- PMID: 37132605
- PMCID: PMC10824235
- DOI: 10.1089/ars.2022.0179
Novel Insights on Ferroptosis Modulation as Potential Strategy for Cancer Treatment: When Nature Kills
Abstract
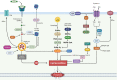
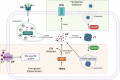
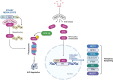
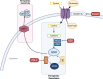
Significance: The multifactorial nature of the mechanisms implicated in cancer development still represents a major issue for the success of established antitumor therapies. The discovery of ferroptosis, a novel form of programmed cell death distinct from apoptosis, along with the identification of the molecular pathways activated during its execution, has led to the uncovering of novel molecules characterized by ferroptosis-inducing properties. Recent advances: As of today, the ferroptosis-inducing properties of compounds derived from natural sources have been investigated and interesting findings have been reported both in vitro and in vivo. Critical Issues: Despite the efforts made so far, only a limited number of synthetic compounds have been identified as ferroptosis inducers, and their utilization is still limited to basic research. In this review, we analyzed the most important biochemical pathways involved in ferroptosis execution, with particular attention to the newest literature findings on canonical and non-canonical hallmarks, together with mechanisms of action of natural compounds identified as novel ferroptosis inducers. Compounds have been classified based on their chemical structure, and modulation of ferroptosis-related biochemical pathways has been reported. Future Directions: The outcomes herein collected represent a fascinating starting point from which to take hints for future drug discovery studies aimed at identifying ferroptosis-inducing natural compounds for anticancer therapies. Antioxid. Redox Signal. 40, 40-85.
Keywords: anticancer strategies; cancer; ferroptosis; ferroptosis inducers; natural compounds; oxidative stress; phytochemicals.
Conflict of interest statement
No competing financial interests exist.
Figures




References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Research Materials
