CYCLOIDEA-like genes control floral symmetry, floral orientation, and nectar guide patterning
- PMID: 37132634
- PMCID: PMC10396386
- DOI: 10.1093/plcell/koad115
CYCLOIDEA-like genes control floral symmetry, floral orientation, and nectar guide patterning
Abstract
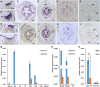
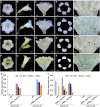
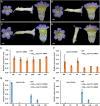
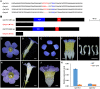
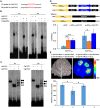
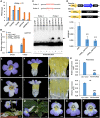
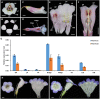
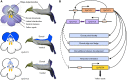
Actinomorphic flowers usually orient vertically (relative to the horizon) and possess symmetric nectar guides, while zygomorphic flowers often face horizontally and have asymmetric nectar guides, indicating that floral symmetry, floral orientation, and nectar guide patterning are correlated. The origin of floral zygomorphy is dependent on the dorsoventrally asymmetric expression of CYCLOIDEA (CYC)-like genes. However, how horizontal orientation and asymmetric nectar guides are achieved remains poorly understood. Here, we selected Chirita pumila (Gesneriaceae) as a model plant to explore the molecular bases for these traits. By analyzing gene expression patterns, protein-DNA and protein-protein interactions, and encoded protein functions, we identified multiple roles and functional divergence of 2 CYC-like genes, i.e. CpCYC1 and CpCYC2, in controlling floral symmetry, floral orientation, and nectar guide patterning. CpCYC1 positively regulates its own expression, whereas CpCYC2 does not regulate itself. In addition, CpCYC2 upregulates CpCYC1, while CpCYC1 downregulates CpCYC2. This asymmetric auto-regulation and cross-regulation mechanism might explain the high expression levels of only 1 of these genes. We show that CpCYC1 and CpCYC2 determine asymmetric nectar guide formation, likely by directly repressing the flavonoid synthesis-related gene CpF3'5'H. We further suggest that CYC-like genes play multiple conserved roles in Gesneriaceae. These findings shed light on the repeated origins of zygomorphic flowers in angiosperms.
© American Society of Plant Biologists 2023. All rights reserved. For permissions, please e-mail: journals.permissions@oup.com.
Conflict of interest statement
Conflict of interest statement. The authors declare that there is no conflict of interest.
Figures

Comment in
-
The extended functions of CYCLOIDEA-like genes in the control of floral architecture.Plant Cell. 2023 Aug 2;35(8):2703-2704. doi: 10.1093/plcell/koad142. Plant Cell. 2023. PMID: 37201286 Free PMC article. No abstract available.
Similar articles
-
Characterization of CYCLOIDEA-like genes in Proteaceae, a basal eudicot family with multiple shifts in floral symmetry.Ann Bot. 2017 Feb;119(3):367-378. doi: 10.1093/aob/mcw219. Epub 2016 Dec 26. Ann Bot. 2017. PMID: 28025288 Free PMC article.
-
Gene duplication and relaxation from selective constraints of GCYC genes correlated with various floral symmetry patterns in Asiatic Gesneriaceae tribe Trichosporeae.PLoS One. 2019 Jan 30;14(1):e0210054. doi: 10.1371/journal.pone.0210054. eCollection 2019. PLoS One. 2019. PMID: 30699126 Free PMC article.
-
An Optimized Transformation System and Functional Test of CYC-Like TCP Gene CpCYC in Chirita pumila (Gesneriaceae).Int J Mol Sci. 2021 Apr 27;22(9):4544. doi: 10.3390/ijms22094544. Int J Mol Sci. 2021. PMID: 33925272 Free PMC article.
-
Floral zygomorphy, the recurring evolution of a successful trait.Bioessays. 2004 Nov;26(11):1175-84. doi: 10.1002/bies.20119. Bioessays. 2004. PMID: 15499590 Review.
-
Advances in Research on the Regulation of Floral Development by CYC-like Genes.Curr Issues Mol Biol. 2023 Mar 2;45(3):2035-2059. doi: 10.3390/cimb45030131. Curr Issues Mol Biol. 2023. PMID: 36975501 Free PMC article. Review.
Cited by
-
BLADE-ON-PETIOLE interacts with CYCLOIDEA to fine-tune CYCLOIDEA-mediated flower symmetry in monkeyflowers (Mimulus).Sci Adv. 2024 Aug 16;10(33):eado4571. doi: 10.1126/sciadv.ado4571. Epub 2024 Aug 14. Sci Adv. 2024. PMID: 39141743 Free PMC article.
-
Genome-Wide Identification and Expression Analysis of TCP Transcription Factors Responding to Multiple Stresses in Arachis hypogaea L.Int J Mol Sci. 2025 Jan 26;26(3):1069. doi: 10.3390/ijms26031069. Int J Mol Sci. 2025. PMID: 39940846 Free PMC article.
-
A single dominant GLOBOSA allele accounts for repeated origins of hose-in-hose flowers in Sinningia (Gesneriaceae).Plant Cell. 2024 Dec 23;37(1):koae283. doi: 10.1093/plcell/koae283. Plant Cell. 2024. PMID: 39422240
-
Evolution and development of complex floral displays.Development. 2024 Nov 1;151(21):dev203027. doi: 10.1242/dev.203027. Epub 2024 Nov 5. Development. 2024. PMID: 39498660 Free PMC article. Review.
-
Non-radiate flowers are associated with truncation of the CYCLOIDEA gene in Scaevola aemula (Goodeniaceae).Sci Rep. 2025 Jul 22;15(1):26645. doi: 10.1038/s41598-025-12463-z. Sci Rep. 2025. PMID: 40696094 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

