Insulin secretory actions of ethanolic extract of Acacia arabica bark in high fat-fed diet-induced obese Type 2 diabetic rats
- PMID: 37133312
- PMCID: PMC10214104
- DOI: 10.1042/BSR20230329
Insulin secretory actions of ethanolic extract of Acacia arabica bark in high fat-fed diet-induced obese Type 2 diabetic rats
Abstract
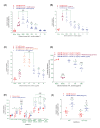
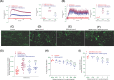
Acacia arabica commonly known as 'babul' has been widely used for the treatment of numerous diseases, including diabetes due to their potential pharmacological actions. The aim of the present study was to investigate the insulinotropic and antidiabetic properties of ethanol extract of Acacia arabica (EEAA) bark through in vitro and in vivo studies in high fat-fed (HFF) rats. EEAA at 40-5000 µg/ml significantly increased (P<0.05-0.001) insulin secretion with 5.6 and 16.7 mM glucose, respectively, from clonal pancreatic BRIN BD11 β-cells. Similarly, EEAA at 10-40 µg/ml demonstrated a substantial (P<0.05-0.001) insulin secretory effect with 16.7 mM glucose from isolated mouse islets, with a magnitude comparable to 1 µM glucagon-like peptide-1 (GLP-1). Diazoxide, verapamil, and calcium-free conditions decreased insulin secretion by 25-26%. The insulin secretory effect was further potentiated (P<0.05-0.01) with 200 µM isobutylmethylxanthine (IBMX; 1.5-fold), 200 µM tolbutamide (1.4-fold), and 30 mM KCl (1.4-fold). EEAA at 40 µg/ml, induced membrane depolarization and elevated intracellular Ca2+ as well as increased (P<0.05-0.001) glucose uptake in 3T3L1 cells and inhibited starch digestion, glucose diffusion, dipeptidyl peptidase-IV (DPP-IV) enzyme activity, and protein glycation by 15-38%, 11-29%, 15-64%, and 21-38% (P<0.05, 0.001), respectively. In HFF rats, EEAA (250 mg/5 ml/kg) improved glucose tolerance, plasma insulin, and GLP-1 levels, and lowered DPP-IV enzyme activity. Phytochemical screening of EEAA revealed the presence of flavonoids, tannins and anthraquinone. These naturally occurring phytoconstituents may contribute to the potential antidiabetic actions of EEAA. Thus, our finding suggests that EEAA, as a good source of antidiabetic constituents, would be beneficial for Type 2 diabetes patients.
Keywords: GLP-1; Insulin; glucose; obesity; phytoconstituents; type 2 diabetes.
© 2023 The Author(s).
Conflict of interest statement
The Animal Welfare and Ethical Review Board (AWERB) at Ulster University approved the use of animals for research in May 2018. The UK Home Office issued project/personal license numbers PIL1822 and PPL2804 in May 2016 and February 2017, respectively, under which the experiments were conducted. All experiments were performed in the Biomedical and Behavioral Research Unit (BBRU) at Ulster University, Coleraine, U.K. in accordance with the UK Act 1986 and EU Directive 2010/63EU, and necessary measures were taken to make sure no animals were harmed throughout the course of the research. Samples of blood were obtained from the cut tail tips of live animals; they were not executed.
The authors declare that there are no competing interests associated with the manuscript.
Figures

References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

